��Ŀ����
A��B��C��D��E����ǰ������ԭ�������������������Ԫ�ء�A��Dͬ�����������ֳ���������DA2��DA3����ҵ�ϵ������C2A3��ȡ����C��B��E��������ֻ��2�������⣬�������ȫ������Eλ��Ԫ�����ڱ���ds�����ش��������⣺
��1��B��C�е�һ�����ܽϴ����_________����̬Dԭ�Ӽ۵��ӵĹ������ʽΪ____________��
��2��DA2���ӵ�VSEPRģ����____________��H2A��H2D�۷е�ߵö��ԭ����____________��
��3��ʵ����C����Ԫ���γɻ������ʵ�����ΪC2Cl6�������ģ����ͼ��ʾ����֪C2Cl6 �ڼ���ʱ���������������NaOH��Һ��Ӧ������Na[C(OH)4]��

�� C2Cl6����____________���壨������ͣ�������Cԭ�ӵ��ӻ��������Ϊ____________�ӻ���
�� [C(OH)4]���д��ڵĻ�ѧ����___________��
��4����ҵ���Ʊ�B�ĵ����ǵ������B���Ȼ�������ǵ��BA��ԭ����_____________��
��5��B��C�ķ����ᄃ���ֱܷ���2957 kJ��mol-1��5492 kJ��mol-1���������ܴ��ԭ����____________��
��6��D��E���γɻ����ᄃ��ľ�����ͼ��ʾ��

�� �ڸþ����У�E����λ��Ϊ______________��
�� ��֪�þ������ܶ�Ϊ�� g/cm3������������Dԭ��֮��ľ���Ϊ_________ pm���г�����ʽ���ɣ���
��ҵ������������Ҫ�ɷ�ΪAl2O3������Fe2O3��SiO2�����ʣ���ȡ��ˮ�Ȼ�����һ�ֹ�������ʾ�����£�
��֪��
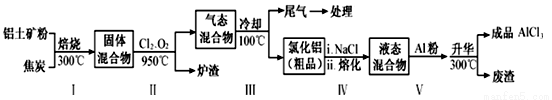
���� | SiCl4 | AlCl3 | FeCl3 | FeCl2 |
�е�/�� | 57.6 | 180�������� | 300�������� | 1023 |
��1��������б���ʹ����ˮ�ֻӷ���������Ŀ���࣬��������_________________��ֻҪ��д��һ�֣���
��2�������������ͨ����������������Ӧ�������ԭ�������ȹ��ĵ�����________________��
��3��������̼�������ݷ���������V�м������۵�Ŀ����________________��
��4����ȡ��Fe2O3��Al2O3������0.2000g�������ܽ���pH=2.0������Һ�У�50�����ң����Իǻ�ˮ����Ϊָʾ������0.02000 mol/L EDTA����Һ�ζ������е�Fe3+����ȥ18.00 mL��Ȼ����Һ����pH=3.5����������EDTA����Һ25.00 mL����������У�ʹAl3+��EDTA��ȫ��Ӧ���ٵ���ҺpH=4.5����PAN��1-��2-���ż����-2-���ӣ�Ϊָʾ����������CuSO4����Һ��ÿ������CuSO4��5H2O 0.005000g�����ζ�����ȥ8.00 mL������������Fe2O3��Al2O3������������________________��д��������̣�
����֪��EDTA��Fe3+��Al3+��Cu2+�������ʵ���֮��1��1���з�Ӧ��


 2NH3(g)����H<0��673K��30MPa��n(NH3)��n(H2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ������������ȷ���ǣ� ��
2NH3(g)����H<0��673K��30MPa��n(NH3)��n(H2)��ʱ��仯�Ĺ�ϵ����ͼ��ʾ������������ȷ���ǣ� ��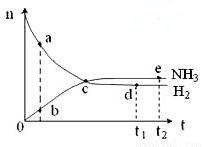
 2NO2 ��g�������н��۲���˵��������Ӧ�ڸ��������Ѿ��ﵽƽ��״̬���ǣ�������
2NO2 ��g�������н��۲���˵��������Ӧ�ڸ��������Ѿ��ﵽƽ��״̬���ǣ������� 

 x Q��g��+3R��g�����÷�Ӧ��ƽ��ʱ������2.4mol R�������Q��Ũ��Ϊ0.4mol/L�������й�������ȷ���ǣ��� ����
x Q��g��+3R��g�����÷�Ӧ��ƽ��ʱ������2.4mol R�������Q��Ũ��Ϊ0.4mol/L�������й�������ȷ���ǣ��� ����