��Ŀ����
A��B��C��D��E����ѧ��ѧ�������ʣ��������ǵ�Ԫ�ص�ԭ�������ֱ�Ϊ����b�� c��d���֣���3 ��a+b�� = 2 ��a+c�� = 3 ��d-a����X��Y��Z��M��N��W��H��K�dz��������X��B��C�Ļ��ϲ������֮��������ת����ϵ��ͼ�з�Ӧ��Ͳ����е�H2O����ȥ����
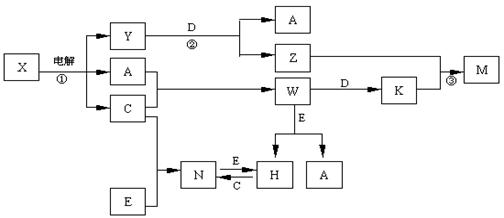
��1��Y�ĵ���ʽΪ ������D���ʵ�Ԫ�������ڱ���λ�� ���� �塣
��2����Ӧ�۵����ӷ���ʽΪ E����������D��һ�������·�Ӧ�Ļ�ѧ����ʽΪ�� ��
��3������N�����¼��ӷ���183�������������������е��л��ܼ���H2O�У��ݴ��ж�
NΪ �;��塣
��4��25��ʱ��PH��5��W��N��ˮ��Һ����H2O���������H������Ũ��֮��Ϊ ��
��1��![]() ��������A
��������A
��2��Al3++3AlO2��+6H2O = 4Al��OH��3
Fe2O3+2Al ![]() 2Fe + Al2O3 ��Fe2O3��д��FeO��Fe3O4����ƽҲ���ԣ�
2Fe + Al2O3 ��Fe2O3��д��FeO��Fe3O4����ƽҲ���ԣ�
��3������
��4��1��104
����:

��ϰ��ϵ�д�
�����Ŀ
��֪A��B��C��D��E�Ƕ�����ԭ�������������������Ԫ�أ�Aԭ����Ԫ�����ڱ���ԭ�Ӱ뾶��С��B��Eͬ���壬��E��ԭ��������B��������C��D�ǽ��������ǵ����������������ˮ������˵������ȷ���ǣ�������
| A�������ӵİ뾶��C��D��E��B | B����ҵ�ϳ��õ�ⷨ�Ƶ�C��D�ĵ��� | C���ȶ��ԣ�A2B��A2E | D������D������ұ��ijЩ���۽��� |

 2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
2DB3����3.2gDB2��ȫת��ΪDB3����ʱ����akJ��1mol DB3������ȫת��Ϊ��ˮ�������bkJ��������33.6L DB2��ȫ�������������Ӧ����
