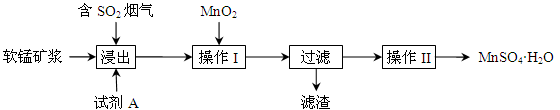
��Ŀ����
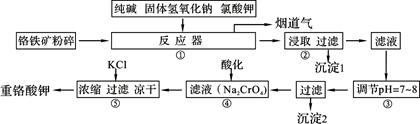
��ҵ�ϳ��ø�������Ч�ɷ�ΪFeO��Cr2O3����Ҫ����ΪSiO2��Al2O3��Ϊԭ�������ظ���أ�K2Cr2O7����ʵ����ģ�ҵ���ø��������ظ���ص���Ҫ������������ͼ���漰����Ҫ��Ӧ�ǣ�6FeO��Cr2O3��24NaOH��7KClO3=12Na2CrO4��3Fe2O3��7KCl��12H2O���Իش��������⣺
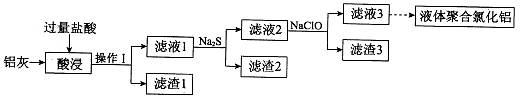
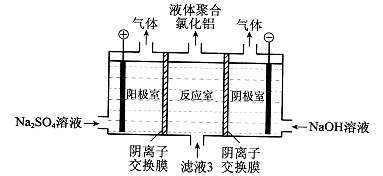
��1��������Һ���������ӵļ��鷽���� ��
��2������۱�����������Ϊ�������ӷ��ţ� ��
��3���ڷ�Ӧ�����У����������봿�Ӧ�Ļ�ѧ����ʽΪ�� ��
��4���̵����е�CO2����H2�ϳɼ״���CH3OH��H2��ȼ���ȷֱ�Ϊ����H=��725.5 kJ/mol����H=��285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��5��2011�����������ĸ���Ⱦ�¼���˵��������������ˮ���������ŷŶ��������滷���м����Σ������ⷨ�Ǵ�������Ⱦ��һ�ַ�������������������ʯī��������⺬Cr2O72-�����Է�ˮ��һ��ʱ������Fe(OH)3��Cr(OH)3������
��д����ⷨ������ˮ���ܷ�Ӧ�����ӷ���ʽ ��
����֪Cr(OH)3��Ksp=6.3��10�C31�����ر�ˮ�����������ֵ��0.1 mg/L��Ҫʹ��Һ��c(Cr3+)�������ϵر�ˮ��ֵ���������Һ��c(OH-)�� mol/L��ֻд�������ʽ����

��1��������Һ���������ӵļ��鷽���� ��
��2������۱�����������Ϊ�������ӷ��ţ� ��
��3���ڷ�Ӧ�����У����������봿�Ӧ�Ļ�ѧ����ʽΪ�� ��
��4���̵����е�CO2����H2�ϳɼ״���CH3OH��H2��ȼ���ȷֱ�Ϊ����H=��725.5 kJ/mol����H=��285.8 kJ/mol��д����ҵ����CO2��H2�ϳ�CH3OH���Ȼ�ѧ����ʽ�� ��
��5��2011�����������ĸ���Ⱦ�¼���˵��������������ˮ���������ŷŶ��������滷���м����Σ������ⷨ�Ǵ�������Ⱦ��һ�ַ�������������������ʯī��������⺬Cr2O72-�����Է�ˮ��һ��ʱ������Fe(OH)3��Cr(OH)3������
��д����ⷨ������ˮ���ܷ�Ӧ�����ӷ���ʽ ��
����֪Cr(OH)3��Ksp=6.3��10�C31�����ر�ˮ�����������ֵ��0.1 mg/L��Ҫʹ��Һ��c(Cr3+)�������ϵر�ˮ��ֵ���������Һ��c(OH-)�� mol/L��ֻд�������ʽ����
��1����ɫ��Ӧ ����2��SiO32-��AlO2-��
��3��Na2CO3��SiO2 Na2SiO3��CO2����
Na2SiO3��CO2����
��4��CO2(g)��3H2(g) = CH3OH(l)��H2O(l) ��H=��131.9 kJ/mol��
��5���� 6Fe��Cr2O72-��2H+��17H2O = 6Fe(OH)3����2Cr(OH)3����6H2����
��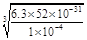 ����
����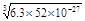 ��
�� ����
����
��3��Na2CO3��SiO2
 Na2SiO3��CO2����
Na2SiO3��CO2������4��CO2(g)��3H2(g) = CH3OH(l)��H2O(l) ��H=��131.9 kJ/mol��
��5���� 6Fe��Cr2O72-��2H+��17H2O = 6Fe(OH)3����2Cr(OH)3����6H2����
��
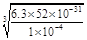 ����
����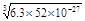 ��
�� ����
���������������1��������Һ����������K+�ļ��鷽��������ɫ��Ӧ�����顣������ȡ����������˿��Pt˿��������ϴ�Ӻ��ھƾ��ƵĻ��������գ�����������ɫ��ͬʱպȡ����Һ���ڻ��������գ�������ɫCo�������۲�������ɫ����������ɫ����֤������K+����2���������������NaOH��Na2CO3�ȼ������ʣ�SiO2��Al2O3������Ӧ��ΪNa2SiO3��AlO2������������Һ��pH��7��8ʱ����ת��ΪH2SiO3��Al(OH)3��������˲���۱�����������ΪSiO32-��AlO2-����3���ڷ�Ӧ�����У����������봿�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3��SiO2
 Na2SiO3��CO2����(4)CH3OH��H2��ȼ�յ��Ȼ�ѧ����ʽΪ���� CH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H=��725.5 kJ/mol ; ��H2(g)+ 1/2O2(g)=H2O(l) ��H=��285.8 kJ/mol. �ڡ�3���١������ɵ�CO2(g)��3H2(g) = CH3OH(l)��H2O(l) ��H=��131.9 kJ/mol����5���ٸ��ݵ����غ㡢����غ㼰�����غ㶨�ɿɵõ�⺬Cr2O72-�����Է�ˮ���ܷ�Ӧ�����ӷ���ʽΪ��6Fe��Cr2O72-��2H+��17H2O = 6Fe(OH)3����2Cr(OH)3����6H2���������ر�ˮ�����������ֵ��0.1 mg/L ��c(Cr3+) =1��10-4g��52g/mol/L=
Na2SiO3��CO2����(4)CH3OH��H2��ȼ�յ��Ȼ�ѧ����ʽΪ���� CH3OH(l)+3/2O2(g)=CO2(g)+2H2O(l) ��H=��725.5 kJ/mol ; ��H2(g)+ 1/2O2(g)=H2O(l) ��H=��285.8 kJ/mol. �ڡ�3���١������ɵ�CO2(g)��3H2(g) = CH3OH(l)��H2O(l) ��H=��131.9 kJ/mol����5���ٸ��ݵ����غ㡢����غ㼰�����غ㶨�ɿɵõ�⺬Cr2O72-�����Է�ˮ���ܷ�Ӧ�����ӷ���ʽΪ��6Fe��Cr2O72-��2H+��17H2O = 6Fe(OH)3����2Cr(OH)3����6H2���������ر�ˮ�����������ֵ��0.1 mg/L ��c(Cr3+) =1��10-4g��52g/mol/L= ��10-4 mol/L .Cr(OH)3��Ksp=6.3��10�C31����c(Cr3+)��c3(OH-)��6.3��10�C31;
��10-4 mol/L .Cr(OH)3��Ksp=6.3��10�C31����c(Cr3+)��c3(OH-)��6.3��10�C31;c3(OH-)��6.3��10�C31��
 ��10-4=6.3��52��10-27.����c(OH-)=
��10-4=6.3��52��10-27.����c(OH-)=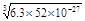 .
.
��ϰ��ϵ�д�
�����Ŀ