��Ŀ����
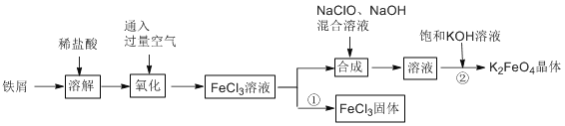
����Ŀ���Ȼ���������ض��dz�����ˮ��������ij��ȤС������мΪԭ��ģ�ҵ���Ʊ��Ȼ�������һ�������Ʊ�������ص��������£�
��ش��������⣺
(1)����������ͨ����������������������������е��ŵ���__________________________________��
(2)���ϳ���������Na2FeO4�����ӷ���ʽΪ__________________________________��
(3)Ϊ�˼�����������������������Һ���Ƿ���Fe2+��ijͬѧȡ������Һ���Թ��У�ѡ�������Լ����ԴﵽĿ�ĵ���_______(����ĸ)��
a.KSCN��Һb.NaOH��Һc.K3[Fe(CN)6]��Һd.������Һ e.����KMnO4��Һ
(4)���̢���ȡFeCl3����ľ������������________________________________________����ʹ6.5 mol/LFeCl3������Һ������Fe(OH)3���������Һ��pHС��_______{��֪��ʵ�������£�Ksp[(Fe(OH)3]=6.5��10-36��Kw=1.1��10-13}
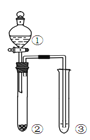
(II)���ļ��Ȼ��[(CH3)4NCl]ˮ��ҺΪԭ�ϣ�ͨ����ⷨ�����Ʊ��ļ��������[(CH3)4NOH]��װ����ͼ��ʾ��

(1)�ռ���(CH3)4NOH��������______(��a��b��c��d)��
(2)д�������ܷ�Ӧ(��ѧ����ʽ)___________________________��
���𰸡�ѡ��ͨ��������á��������ж�����Ⱦ����2Fe3++3ClO-+10OH-==2FeO42-+3C1-+5H2Oc��HCl����������FeCl3��Һ��ȡFeCl3 1d2(CH3)4NCl+2H2O![]() 2(CH3)4NOH+H2��+Cl2��
2(CH3)4NOH+H2��+Cl2��
��������
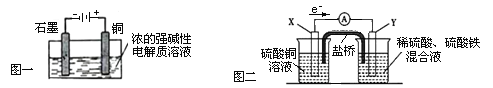
(1) ��Ϊ�������ö������ж�����Ⱦ��������������������ͨ����������������������������еĸ�����ŵ㡣�𰸣�ѡ��ͨ��������á��������ж�����Ⱦ������
(2)���ϳ�Һ���к����Ȼ������ټ���������ƺ��������ƵĻ��Һ����Ӧ����Na2FeO4���Ȼ��ƺ�ˮ����Ӧ�����ӷ���ʽΪ2Fe3++3ClO-+10OH-==2FeO42-+3C1-+5H2O��
(3)��Ϊ�����Һ�к���Fe3+ ��Fe2+�����Լ��룺a.KSCN ��Һ������֤Fe2+�Ĵ��ڣ� b.NaOH ��Һ ��Fe3+�������ɫ����������������Ӱ��۲�Fe2+��Ӧ���������Բ�����NaOH ��Һ���� c.K3[Fe(CN)6]��Һ���� Fe2+�������ɫ���������Կ����� K3[Fe(CN)6]��Һ���� d.������Һ��Fe3+����ɫ����Fe2+���������Բ����ñ�����Һ����e����K MnO4��ҺҲ�����ڼ�����Һ���Ƿ���Fe2+��������Һ�к���Cl����Ҳ����KMnO4������Ӧ��ʹ����ɫ�����Բ������������Ϊ��c��
(4)Ϊ��ֹFe3+��ˮ�⣬����Һ������FeCl3������Ҫ��HC1����������FeCl3 ��Һ����֪c(Fe3+)=6.4 mol/L��Ksp[(Fe(OH)3]=8.5��10-36������c3(OH��)=![]() =
= ![]() =1.33��10-36��c(OH��)=1.1��10-12 mol/L�������Һ�е�c(H+)=0.1 mol/L����pH=1�������������Һ��pHС��1��
=1.33��10-36��c(OH��)=1.1��10-12 mol/L�������Һ�е�c(H+)=0.1 mol/L����pH=1�������������Һ��pHС��1��
��1����ʯīΪ�缫����ļ��Ȼ����Һ�Ʊ��ļ�������泥���Ҫ���������ӣ��������������õ����������������缫�����������������ӣ������ռ���(CH3)4NOH������������������d�ڡ��𰸣�d��
��2����Ϸ�Ӧ���������Ĺ�ϵ��֪�����������ɲ���Ϊ�ļ�������李��������������ݴ�д����ⷴӦ�Ļ�ѧ����ʽ��2(CH3)4NCl+2H2O![]() 2(CH3)4NOH+H2��+Cl2����
2(CH3)4NOH+H2��+Cl2����

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���( )
ѡ�� | �� | �� | �� | ʵ����� | ʵ��װ�� |
A | ϡ���� | Na2S | AgNO3��AgCl����Һ | Ksp(AgCl)��Ksp(Ag2S) |
|
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
C | ϡ���� | Na2SO3 | Ba(NO3)2 ��Һ | SO2������Ա��ξ��������ɰ�ɫ���� | |
D | Ũ���� | Na2CO3 | Na2SiO3��Һ | ���ԣ����̼����� |
A. A B. B C. C D. D
����Ŀ��POCl3�������뵼����Ӽ������άԭ�ϣ�ʵ�����Ʊ�POCl3���ⶨ��Ʒ������ʵ��������£�
I.ʵ�����Ʊ�POCl3��������������Һ̬PCl3����ȡPOCl3��ʵ��װ��(���ȼ��г�������)��ͼ��

���ϣ���Ag++SCN-=AgSCN����Ksp(AgCl)>Ksp(Ag SCN)��
��PCl3��POCl3�������Ϣ���±���
���� | �۵�/�� | �е�/�� | ��Է������� | ���� |
PCl3 | -112.0 | 76.0 | 137.5 | �����ܣ���Ϊ��ɫҺ�壬��ˮ�����ҷ�Ӧ���ɺ�������Ȼ��� |
POCl3 | 2.0 | 106.0 | 153.5 |
(1) װ��B�е������ǹ۲����������ٺ�______________��_______________ ������ܵ�������_____________
(2)��Ӧ�¶�Ҫ������60��65�棬ԭ���ǣ�________________________________________________
II.�ⶨPOCl3��Ʒ�ĺ�����
ʵ�鲽�裺
���Ʊ�POCl3ʵ�����������ƿ�е�Һ����ȴ�����£�ȷ��ȡ30.7 g��Ʒ(���ʲ�����Ԫ��)������ʢ��60.00 mL����ˮ��ˮ��ƿ��ҡ������ȫˮ�⣬��ˮ��Һ���100.00 mL ��Һ��
��ȡ10.00 mL��Һ����ƿ�У�����10.00 mL 3.2 moI/L AgNO3����Һ��
�ۼ�����������������ҡ����ʹ�������汻�л��︲�ǡ�
����XΪָʾ������0.2 moI/L KSCN��Һ�ζ�������AgNO3��Һ���ﵽ�ζ��յ�ʱ����ȥ10.00 mL KSCN��Һ��
(3)ʵ������5.0 moI/L AgNO3 ����100 mL 3.2 moI/L AgNO3����Һ����ʹ�õ��������ձ��Ͳ��������_____________________
(4)��������������������Ľ����²������_____(��ƫ�ߡ�ƫ�ͻ���Ӱ��)
(5)�������X����ѡ��_____��
(6)��Ӧ��POCl3�������ٷֺ���Ϊ_____��ͨ��_____(�����)������߲�Ʒ�Ĵ��ȡ�