��Ŀ����
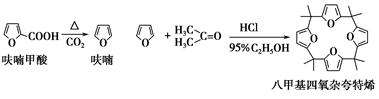
��ૼ������ȵõ�����ͪ���������������ϣ��ɵõ��˼������ӿ���ϩ���й�ʵ��ԭ�����������£�
����1����Ʊ�
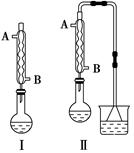
��Բ����ƿ�з���4.5 gૼ��ᣨ100 ��������ૼ�����133 �����ڣ�230��232 ����ڣ����ڴ��¶������ȣ�����ͼ��װ���������ȴ�����ʹૼ�������ۻ���Ȼ����ڼ���ǿ�ȣ��������У���ૼ������ȷ�Ӧ��ϣ�ֹͣ���ȡ�����ɫҺ��ૣ��е㣺31��32 �棬������ˮ����
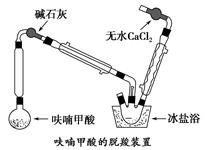
����2��������˼������ӿ���ϩ�ĺϳ�
��25 mL��ƿ�м���2.7 mL 95%�Ҵ���1.35 mLŨ���ᣬ���ȣ��ڱ�ԡ������5 �����£�Ȼ��3.3 mL��ͪ��1.35 mLĻ��ҺѸ�ٵ�����ƿ�У���ֻ��ȣ���ԡ��ȴ�����õ�һ��ɫ��״���塣���ˣ�����3 mL��ˮ�Ҵ�ϴ�ӣ��ñ��ؽᾧ���ð�ɫ�ᾧ�˼������ӿ���ϩ��
��1������1���ô���ټ��ȣ�����ҪĿ����____________________________��
��2��װ��ͼ�м�ʯ�ҵ�������__________________________________________��
��ˮ�Ȼ��Ƶ�������________________________________________________��
��3������װ�����ñ���ԡ��Ŀ����_____________________________________��
��4���ϳɰ˼������ӿ���ϩ���������Ŀ����_________________________��
��5��ȷ�۲�ƷΪ�˼������ӿ���ϩ����ͨ���ⶨ�е㣬���ɲ��õļ�ⷽ����__________________________________________________________��

����1����Ʊ�
��Բ����ƿ�з���4.5 gૼ��ᣨ100 ��������ૼ�����133 �����ڣ�230��232 ����ڣ����ڴ��¶������ȣ�����ͼ��װ���������ȴ�����ʹૼ�������ۻ���Ȼ����ڼ���ǿ�ȣ��������У���ૼ������ȷ�Ӧ��ϣ�ֹͣ���ȡ�����ɫҺ��ૣ��е㣺31��32 �棬������ˮ����

����2��������˼������ӿ���ϩ�ĺϳ�
��25 mL��ƿ�м���2.7 mL 95%�Ҵ���1.35 mLŨ���ᣬ���ȣ��ڱ�ԡ������5 �����£�Ȼ��3.3 mL��ͪ��1.35 mLĻ��ҺѸ�ٵ�����ƿ�У���ֻ��ȣ���ԡ��ȴ�����õ�һ��ɫ��״���塣���ˣ�����3 mL��ˮ�Ҵ�ϴ�ӣ��ñ��ؽᾧ���ð�ɫ�ᾧ�˼������ӿ���ϩ��
��1������1���ô���ټ��ȣ�����ҪĿ����____________________________��
��2��װ��ͼ�м�ʯ�ҵ�������__________________________________________��
��ˮ�Ȼ��Ƶ�������________________________________________________��
��3������װ�����ñ���ԡ��Ŀ����_____________________________________��
��4���ϳɰ˼������ӿ���ϩ���������Ŀ����_________________________��
��5��ȷ�۲�ƷΪ�˼������ӿ���ϩ����ͨ���ⶨ�е㣬���ɲ��õļ�ⷽ����__________________________________________________________��
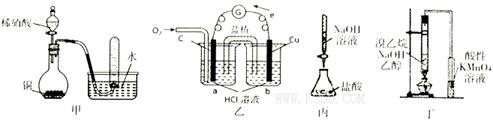
��1����ֹ��������࣬��������ܵĶ�������Ⱦ����
��2���������ɵ�CO2����ֹˮ�������룬�����
��3����ӷ�������ԡ���ٻӷ�����߲���
��4��������
��5������Ʒ�ĺ˴Ź������ͺ������
��2���������ɵ�CO2����ֹˮ�������룬�����
��3����ӷ�������ԡ���ٻӷ�����߲���
��4��������
��5������Ʒ�ĺ˴Ź������ͺ������
��1���������Ϣ��֪ૼ�����100 ��ʱ��������2����ʯ����Ϊ������ૼ������Ȳ�����CO2����ˮ�Ȼ��ƽӿգ���Ϊ�˷�ֹ�����е�ˮ�������롣��3��е�31��32 �棬�ӷ����ñ���ԡ���ٻӷ�����4���ɷ�Ӧԭ��֪��HCl����������5������л������ͨ���˴Ź������ͺ�����ס�

��ϰ��ϵ�д�
�����Ŀ
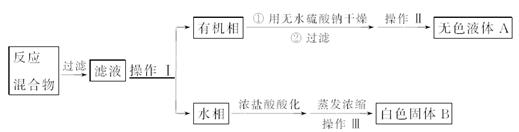
 Fe(SCN)3+3KCl��Һƽ����ϵ�м�����������KCl
Fe(SCN)3+3KCl��Һƽ����ϵ�м�����������KCl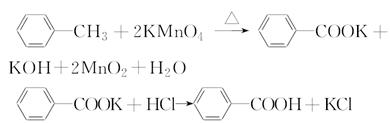
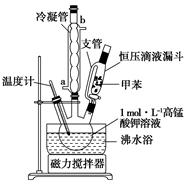
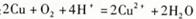
 CH3CH2CHCH2����H2O
CH3CH2CHCH2����H2O