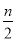��Ŀ����
��ѧƽ�ⳣ��(K)������ĵ���ƽ�ⳣ��(Ka)����������ܶȻ�����(Ksp)���ж��������ʻ�仯����Ҫ��ƽ�ⳣ�������й�����Щ������˵���У���ȷ����(����)
A��ƽ�ⳣ���Ĵ�С���¶ȡ�Ũ�ȡ�ѹǿ���������й�
B�����¶�����ʱ������ĵ���ƽ�ⳣ��Ka��С
C��Ksp(AgCl)>Ksp(AgI)���ɴ˿����ж�AgCl(s)��I��(aq)=AgI(s)��Cl��(aq)�ܹ�����
D��Ka(HCN)<Ka(CH3COOH)��˵�����ʵ���Ũ����ͬʱ������������Աȴ���ǿ
C
��������ƽ�ⳣ���Ĵ�Сֻ���¶��йأ�A���������ĵ���ƽ��Ϊ���ȷ�Ӧ�������¶ȣ�����ƽ�ⳣ������B�������ƽ�ⳣ��Խ�����������Խǿ������Խǿ��D�����

��֪���������������·�Ӧ:2Cu+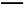 Cu2++Cu�����ڷ�Ӧ�¶Ȳ�ͬ,��������ԭ����ͭʱ,���ܲ���Cu��Cu2O,���߶��Ǻ�ɫ���塣һͬѧ��ij����������ԭ����ͭʵ�����õĺ�ɫ�������������֤,ʵ�������ʵ�������¼����:
Cu2++Cu�����ڷ�Ӧ�¶Ȳ�ͬ,��������ԭ����ͭʱ,���ܲ���Cu��Cu2O,���߶��Ǻ�ɫ���塣һͬѧ��ij����������ԭ����ͭʵ�����õĺ�ɫ�������������֤,ʵ�������ʵ�������¼����:
���� �Լ� | ϡ���� | Ũ���ᡢ ���� | ϡ���� | Ũ���� |
ʵ�� ���� | ��ɫ����� ��ɫ��Һ | ��ɫ���� | ��ɫ����� ��ɫ��Һ | ����ɫ���� ����ɫ��Һ |
�ɴ��Ƴ�����������ԭ����ͭʵ��IJ�����(����)
A.Cu B.һ����Cu,������Cu2O
C.Cu2O D.һ����Cu2O,������Cu
����ѪҺ��Ca2�����ӵ�Ũ��һ�����mg/mL����ʾ���������IJ����[(NH4)2C2O4]��Һ�������������(CaC2O4)���������˲���Ƴ���ϴ�Ӻ�����ǿ��ɵò���(H2C2O4)������KMnO4��Һ�ζ���ʹ����ת����CO2�ݳ���
�Իش�
(1)����Ҫ80 mL 0.02 mol��L��1��KMnO4��Һ�����еζ�����������Һʱ��Ҫ�IJ����������ձ�����������______________________������ʱ�� KMnO4��ҺӦ��ǿ���ữ����ʵ��ѡ��________���ữ������ѡ��HNO3�ữ����������________(����ƫ������ƫС������������)��
(2)������KMnO4��Ӧ�����ӷ���ʽΪ______________________________________
(3)�ζ�ʱ����������___________________________________
����ȷ����Ӧ�ﵽ�յ㡣
(4)�ζ���ʵ������������ʾ��
ʵ���� | ����ѪҺ�����/mL | ����KMnO4��Һ�����/mL |
1 | 20.00 | 11.95 |
2 | 20.00 | 13.00 |
3 | 20.00 | 12.05 |
�������㣬ѪҺ��Ʒ��Ca2�����ӵ�Ũ��Ϊ________mg/mL��
(5)�ζ��ķ���������к͵ζ��������ζ�����ϵζ��ȡ������ζ����õ�ָʾ����������һ�ֳ���������AgNO3����Һ�ⶨCl��Ϊ����
�յ�ǰ��Ag����Cl��=AgCl (��ɫ)
�յ�ʱ��2Ag����CrO42��=Ag2CrO4(ש��ɫ)
������ΪAgCl�ܽ�ȱ�Ag2CrO4��________��Ե�ʡ�