��Ŀ����
12��ͭ�ж��ֻ����������ͭ��Cu2O�����Ȼ���ͭ��CuCl�����Ȼ�ͭ��CuCl2����CuSO4�ȣ�������м��㣺
��1��������Һ��������ͭ����ʯ�Һ�ˮ���Ƴɵ�ɱ��������ͬ�������Ҫ���Ʋ�ͬ�ı����������ư�������CuSO4��CaO��H2O=1��2��200�IJ�����Һ50Kg��
��Ҫ��CuSO4•5H2O385 g��CaO8.8mol
��2��ij�����Ծ���ͭ����Ҫ�ɷ�ΪCu2S��Ϊԭ��ұ������ͭ������ͭ���к�23%���ʣ��վ�����Cu��97.5%�Ĵ�ͭ42t��
��֪���ܷ�Ӧʽ Cu2S+O2$\stackrel{����}{��}$2Cu+SO2
�վ��辫��ͭ��66.5 t �վ���SO2��״���������7.17��106 L
��3��ӡˢ��·���ͭ�ܱ�FeCl3����Һ��ʴ����ӡˢ��·�����200mL FeCl3��Һ�У���11.2g Cu����ʴ����ȡ��ӡˢ��·�壬����Һ�м���11.2g���ۣ���ַ�Ӧ��Һ�л���4.8g���������ԭFeCl3��Һ�����ʵ���Ũ�ȣ�
��4���Ʊ�ͭ��ij�����ᄃ�壮ȡ5.12g Cu��14.5mol/L HNO3 15mL��6.0mol/L HC1 50mL����Ϻ�Cu��ȫ��Ӧ����Ӧ����Һ����ˮ54.32g���پ�ˮԡ����������42gˮ����ȴ��20�沢���ˣ��õ�8.12g���壮ͨ�������ƶϴ˾���Ļ�ѧʽ��
��֪��20���ܽ�� CuC12•2H2O 73g/100g H2O Cu��NO3��2•3H2O 125g/100g H2O��
���� ��1������������CuSO4��CaO��H2O=1��2��200�������CuSO4��CaO�������ٸ���n��CuSO4•5H2O��=n��CuSO4�������m=nM���CuSO4•5H2O����������n=$\frac{m}{M}$����CaO�����ʵ�����
��2�����վ�����Cu��97.5%�Ĵ�ͭ42t��Ҫ������23%�ľ���ͭ������Ϊx�����ݹ�ϵʽ���м��㣬�������غ�����վ���SO2��״���������
��3�����ݷ�Ӧ��Cu+2FeCl3�TCuCl2+2FeCl2��Fe+CuCl2=FeCl2+Cu��4.8g������Ϊͭ�����ݷ���ʽ�г�����ͭ�Ĺ�ϵ������⣻
��4��ȡ5.12gCu�����ʵ�����0.08mol������ͭԪ���غ㣬Cu������ȫת��ΪCuCl2•2H2O���壬������13.68g����ȫת��Ϊ Cu��NO3��2•3H2O���壬������19.36g����Ӧ����Һ����ˮ54.32g���پ�ˮԡ����������42g���õ��ı�����Һ�к���ˮ22.32g�������ܽ�ȵĸ�����м��㼴�ɣ�
��� �⣺��1��������Һ50Kg=50000g����������CuSO4��CaO��H2O=1��2��200���ƣ�m��CuSO4��=50000g��$\frac{1}{1+2+200}$��246.3g��n��CuSO4��=$\frac{246.3g}{160g/mol}$��n��CuSO4•5H2O��=n��CuSO4��=$\frac{246.3g}{160g/mol}$��m��CuSO4•5H2O��=nM=$\frac{246.3}{160}��250$g��385g��m��CaO��=50000g��$\frac{2}{1+2+200}$��492.6g��n��CaO��=$\frac{m}{M}$=$\frac{492.6g}{56g/mol}$��8.8mol��
�ʴ�Ϊ��385��8.8��
��2�����辫��ͭ�������Ϊx������Ԫ���غ㣺
Cu2S����������2Cu
160t 128t
x•��1-23%�� 42��97.5%t
����x•77%��42��97.5%t=160t��128t
���x=66.5t
���վ���SO2��״�������Ϊy������Ԫ���غ㣺
2Cu����������Cu2S��������SO2
128t 1 1
42��97.5%t 1 $\frac{y}{22.4}$
����42��97.5%t��$\frac{y}{22.4}$=128t��1
���y=7.17��106��
�ʴ�Ϊ��66.5��7.17��106��
��3����ӡˢ��·�����200mL FeCl3��Һ�У���11.2g Cu����ʴ����Cu+2FeCl3�TCuCl2+2FeCl2������Fe��ǰ����Һ����Cu2+��Fe2+��Cl-�����ܻ���Fe3+������Һ�м���11.2g���ۣ�Fe+CuCl2=FeCl2+Cu����ַ�Ӧ��Һ�л���4.8g���������Fe3+����ôFeǡ�ð�11.2gCuȫ���û��������ﲻ����ΪFeֻ����Cu�����ܽ��ͭ�����ʵ���Ϊ$\frac{11.2g-4.8g}{64g/mol}$=0.1mol��Cu+2FeCl3�TCuCl2+2FeCl2���μӷ�Ӧ��FeCl3���ʵ���Ϊ0.2mol��11.2gFe�����ʵ���Ϊ��$\frac{11.2g}{56g/mol}$=0.2mol��Fe+2FeCl3�T3FeCl2���μӷ�Ӧ��FeCl3���ʵ���Ϊ0.4mol��
����ԭFeCl3��Һ�����ʵ���Ũ��$\frac{0.2mol+0.4mol}{0.2L}$=3mol/L��
��ԭFeCl3��Һ�����ʵ���Ũ��Ϊ3mol/L��
��4�����ʻ�Ϻ�����Ӧ�ı���ʽΪ��Cu+NO3-+H+=Cu2++H2O+NOx��������20���ܽ�ȣ�CuCl2•2H2O 73g/100gH2O��Cu��NO3��2•3H2O��125g/100gH2O������Cu��NO3��2•3H2O���ܽ�ȴ���������ӵ�ʣ������С��������������CuCl2���壬ȡ5.12gCu�����ʵ�����$\frac{5.12g}{64g/mol}$=0.08mol������ͭԪ���غ㣬���ɵ��Ȼ�ͭ������5.12��$\frac{135}{64}$=10.8g����Ӧ����Һ��ˮ54.32g���پ�ˮԡ����������42g���õ��ı�����Һ�к���ˮ12.32g���������ľ����к����Ȼ�ͭ��������S����$\frac{73��\frac{135}{171}}{100+73��\frac{136}{171}}$=$\frac{S}{100g}$����S=50g�������յõ��ľ���Ļ�ѧʽΪ��CuCl2•xH2O������$\frac{10.8-8.12��\frac{135}{135+18x}}{12.32-8.12��\frac{18x}{135+18x}}$=$\frac{50.0}{100}$�����x=3��������������CuCl2•3H2O��
�𣺾���Ļ�ѧʽΪ��CuCl2•3H2O��
���� ���⿼�黯ѧ����ʽ����ؼ��㣬�����ۺϼ�����Ŀ��ע���Ԫ���غ�ĽǶ���д��ϵʽ���㣬��Ŀ�ѶȽϴ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��ѧʵ���Ҳ��ر���������ȷ������� | |
| B�� | ϡ��Ũ����ʱҪ��Ũ��������ע��ˮ���Ҳ��Ͻ��� | |
| C�� | ��ѧʵ���ҿռ��С����ȼ�ױ�����������ʿɻ�� | |
| D�� | һ���ܲ����ж������ʵ���������ʵ�����н��� |
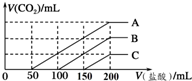 Ũ����ͬ��NaOH��Һ��100mL����A��B��C�����ձ��У��ֱ���������Һ��ͨ�벻������CO2����ַ�Ӧ����������������Һ����μ���0.2mol/L�����ᣬ����CO2���������״���£��������������֮���ϵ��ͼ��ʾ�������ж���ȷ���ǣ�������
Ũ����ͬ��NaOH��Һ��100mL����A��B��C�����ձ��У��ֱ���������Һ��ͨ�벻������CO2����ַ�Ӧ����������������Һ����μ���0.2mol/L�����ᣬ����CO2���������״���£��������������֮���ϵ��ͼ��ʾ�������ж���ȷ���ǣ�������| A�� | ͨ��CO2��A�ձ��е����ʳɷ���Na2CO3 | |
| B�� | B�ձ���ͨ���CO2���Ϊ448 mL | |
| C�� | ԭNaOH��Һ��Ũ��Ϊ0.2 mol/L | |
| D�� | ͨ��CO2��C�ձ������ʳɷֵ����ʵ���֮��Ϊn��NaOH����n��Na2CO3��=1��2 |
| A�� | 2NO2?N2O4 | B�� | 2NO2?2NO+O2 | ||
| C�� | 2HI?H2+I2 | D�� | FeCl3+3KSCN?Fe��SCN��3+3KCl |
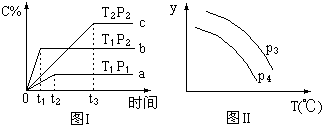 ��ij�ݻ�һ�����ܱ������У������еĿ��淴Ӧ����g��+B��g��?xC��g��������Ӧ���ȣ���ͼ����ʾ�ķ�Ӧ���ߣ����ж϶�ͼ���˵������ȷ���ǣ�T��ʾ�¶ȣ�P��ʾѹǿ��C%��ʾC�������������������
��ij�ݻ�һ�����ܱ������У������еĿ��淴Ӧ����g��+B��g��?xC��g��������Ӧ���ȣ���ͼ����ʾ�ķ�Ӧ���ߣ����ж϶�ͼ���˵������ȷ���ǣ�T��ʾ�¶ȣ�P��ʾѹǿ��C%��ʾC�������������������| A�� | P3��P4��y���ʾA�����ʵ��� | |
| B�� | P3��P4��y���ʾB��������� | |
| C�� | P3��P4��y���ʾ���������ܶ� | |
| D�� | P3��P4��y���ʾ��������ƽ����Է������� |
| A�� | �ڣ��٣��� | B�� | �٣��ڣ��� | C�� | �ۣ��٣��� | D�� | �٨T�ڨT�� |
| ���� | HA | HB | H2C |
| ����ƽ�ⳣ�� ��25�棩 | K1=1.77��10-4 | K1=4.9��10-10 | K1=4.3��10-7 K2=5.6��10-11 |
| A�� | B-+HA��HB+A- | |
| B�� | 2B��+H2C��2HB+C2- | |
| C�� | �к͵��������pH��HA��HB����NaOH����ǰ��С�ں��� | |
| D�� | ���������Ũ�ȵ�NaA��NaB��Һ��������������ǰ�ߴ��ں��� |
| A�� | Na+��Ba2+��NO3-��SO42- | B�� | Cl-��K+��H+��SO42- | ||
| C�� | Na+��Cl-��K+��Ag+ | D�� | Fe2+��SO42-��OH-��Na+ |
| A�� | ���� | B�� | �ҷ��� | C�� | һ���� | D�� | ���Ƚ� |