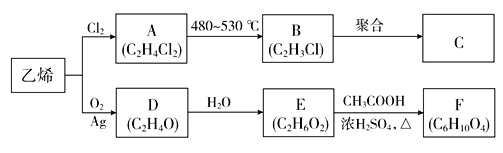
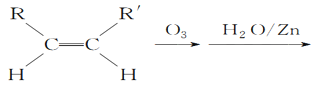
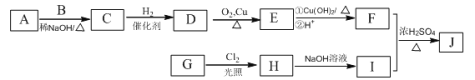
ƒøƒ⁄»ð
°æƒø°ø£®10∑÷£©∫¨ÃºŒÔ÷ µƒº€÷µ–Õ◊™ªØ£¨”–¿˚”⁄°∞ºıú°±∫Õø…≥÷–¯–‘∑¢’𣨔–◊≈÷ÿ“™µƒ—–æøº€÷µ°£«Îªÿ¥œ¬¡–Œ £∫
£®1£©“—÷™CO∑÷◊”÷–ªØ—ߺ¸Œ™C°‘O°£œýπÿµƒªØ—ߺ¸º¸ƒÐ ˝æð»Áœ¬£∫
ªØ—ߺ¸ | H°™O | C°‘O | C=O | H°™H |
E/(kJ°§mol1) | 463 | 1075 | 803 | 436 |
CO(g)£´H2O(g)![]() CO2(g)£´H2(g) ¶§H=___________kJ°§mol1°£œ¬¡–”–¿˚”⁄÷∏þCO∆Ω∫‚◊™ªØ¬ µƒ¥Î ©”–_______________£®ÃÓ±Í∫≈£©°£
CO2(g)£´H2(g) ¶§H=___________kJ°§mol1°£œ¬¡–”–¿˚”⁄÷∏þCO∆Ω∫‚◊™ªØ¬ µƒ¥Î ©”–_______________£®ÃÓ±Í∫≈£©°£
a£Æ‘ˆ¥Û—π«ø b£ÆΩµµÕŒ¬∂»
c£Æ÷∏þ‘≠¡œ∆¯÷–H2Oµƒ±»¿˝ d£Æ π”√∏þ–ߥþªØº¡
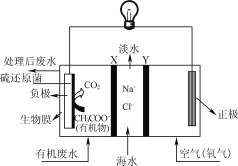
£®2£©”√∂Ë–‘µÁº´µÁΩ‚KHCO3»Ð“∫£¨ø…Ω´ø’∆¯÷–µƒCO2◊™ªØŒ™º◊À·∏˘(HCOO)£¨»ª∫ÛΩ¯“ª≤Ωø…“‘÷∆µ√÷ÿ“™”–ª˙ªØπ§‘≠¡œº◊À·°£CO2∑¢…˙∑¥”¶µƒµÁº´∑¥”¶ ΩŒ™________________£¨»ÙµÁΩ‚π˝≥Ã÷–◊™“∆1 molµÁ◊”£¨—Ùº´…˙≥…∆¯ÃµƒÃª˝£®±Í◊º◊¥øˆ£©Œ™_________L°£
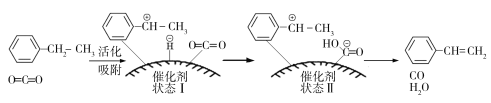
£®3£©““±Ω¥þªØÕ—«‚÷∆»°±Ω““œ©µƒ∑¥”¶Œ™£∫![]() (g)£´CO2(g)
(g)£´CO2(g)![]()
![]() (g)£´CO(g)£´H2O(g)£¨∆‰∑¥”¶¿˙≥ûÁœ¬£∫
(g)£´CO(g)£´H2O(g)£¨∆‰∑¥”¶¿˙≥ûÁœ¬£∫
¢Ÿ”…‘≠¡œµΩ◊¥Ã¨¢Ò____________ƒÐ¡ø£®ÃÓ°∞∑≈≥ˆ°±ªÚ°∞Œ¸ ’°±£©°£
¢⁄“ª∂®Œ¬∂»œ¬£¨œÚ∫„»ð√б’»ð∆˜÷–≥‰»Î2 mol““±Ω∫Õ2 mol CO2£¨∆ º—π«øŒ™p0£¨∆Ω∫‚ ±»ð∆˜ƒ⁄∆¯ÃÂ◊ÐŒÔ÷ µƒ¡øŒ™5 mol£¨““±Ωµƒ◊™ªØ¬ Œ™_______£¨”√∆Ω∫‚∑÷—π¥˙ÃÊ∆Ω∫‚≈®∂»±Ì 浃ªØ—ß∆Ω∫‚≥£ ˝Kp=_______°£[∆¯ÃÂ∑÷—π(p∑÷)=∆¯ÃÂ◊Зπ(p◊Ð)°¡∆¯ÃÂê˝∑÷ ˝]
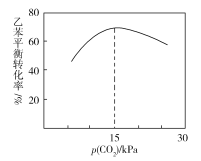
¢€““±Ω∆Ω∫‚◊™ªØ¬ ”Îp(CO2)µƒπÿœµ»Áœ¬ÕºÀ˘ 棨«ÎΩ‚ Õ““±Ω∆Ω∫‚◊™ªØ¬ ÀÊ◊≈p(CO2)±‰ªØ∂¯±‰ªØµƒ‘≠“Ú________________________________________________°£
°æ¥∞∏°ø41 bc 2CO2£´2e£´H2O![]() HCOO£´
HCOO£´![]() ªÚCO2£´2e£´H2O
ªÚCO2£´2e£´H2O![]() HCOO£´OH 5.6 Œ¸ ’ 50% 0.25p0 ÀÊ◊≈CO2—π«ø‘ˆ¥Û£¨CO2≈®∂»‘ˆ¥Û£¨““±Ω∆Ω∫‚◊™ªØ¬ ‘ˆ¥Û£ªCO2—π«øºÃ–¯‘ˆ¥Û£¨ª·‘Ï≥…¥þªØº¡±Ì√Ê““±ΩµƒŒ¸∏Ω¬ œ¬Ωµ
HCOO£´OH 5.6 Œ¸ ’ 50% 0.25p0 ÀÊ◊≈CO2—π«ø‘ˆ¥Û£¨CO2≈®∂»‘ˆ¥Û£¨““±Ω∆Ω∫‚◊™ªØ¬ ‘ˆ¥Û£ªCO2—π«øºÃ–¯‘ˆ¥Û£¨ª·‘Ï≥…¥þªØº¡±Ì√Ê““±ΩµƒŒ¸∏Ω¬ œ¬Ωµ
°æΩ‚Œˆ°ø
£®1£©¶§H=463 kJ°§mol1°¡2£´1075 kJ°§mol1803 kJ°§mol1°¡2436 kJ°§mol1=41 kJ°§mol1°£
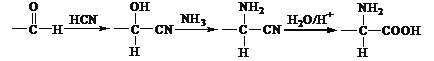
£®2£©CO2◊™ªØŒ™HCOOµ√µΩ2∏ˆµÁ◊”£¨”√OH∆Ω∫‚µÁ∫…£¨µÁº´∑¥”¶ ΩŒ™CO2£´2e£´H2O![]() HCOO£´OHªÚ2CO2£´2e£´H2O
HCOO£´OHªÚ2CO2£´2e£´H2O![]() HCOO£´
HCOO£´![]() £ª—Ùº´µÁΩ‚…˙≥…—ı∆¯£¨µÁΩ‚π˝≥Ã÷–◊™“∆1 molµÁ◊”£¨…˙≥…—ı∆¯µƒÃª˝£®±Í◊º◊¥øˆ£©Œ™5.6 L°£
£ª—Ùº´µÁΩ‚…˙≥…—ı∆¯£¨µÁΩ‚π˝≥Ã÷–◊™“∆1 molµÁ◊”£¨…˙≥…—ı∆¯µƒÃª˝£®±Í◊º◊¥øˆ£©Œ™5.6 L°£
£®3£©¢Ÿ”…‘≠¡œµΩ◊¥Ã¨¢Ò∑¢…˙ªØ—ߺ¸µƒ∂œ¡—£¨–Ë“™Œ¸ ’ƒÐ¡ø°£
¢⁄…Ë““±Ω∑¥”¶¡Àx mol°£
![]() (g)£´CO2(g)
(g)£´CO2(g)![]()
![]() (g)£´CO(g)£´H2O(g)
(g)£´CO(g)£´H2O(g)
n0/mol 2 2 0 0 0
¶§n0/mol x x x x x
[n]/mol 2x 2x x x x
Ω‚µ√£∫4£´x=5
x=1
““±Ωµƒ◊™ªØ¬ Œ™![]() °¡100%=50%
°¡100%=50%
∆Ω∫‚∫Û—π«øŒ™![]() °¡p0=1.25p0£¨Kp=
°¡p0=1.25p0£¨Kp=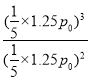 =0.25 p0
=0.25 p0
¢€“ª∂®∑∂Œßƒ⁄£¨p(CO2)‘Ω¥Û£¨Àµ√˜‘⁄‘≠¡œ÷–CO2µƒ≈‰±»‘Ω∏þ£¨‘Ú““±Ω∆Ω∫‚◊™ªØ¬ ‘Ω∏þ£ª∂˛’þ ◊œ»Œ¸∏Ω‘⁄¥þªØº¡±Ì√Ê…œ£¨µ±CO2‘⁄¥þªØº¡±Ì√ÊŒ¸∏Ω¬ π˝∏þ ±£¨ª·‘Ï≥…““±Ω‘⁄¥þªØº¡±Ì√ʵƒŒ¸∏Ω¬ œ¬Ωµ£¨ π““±Ω∆Ω∫‚◊™ªØ¬ ÀÊ◊≈p(CO2)‘ˆ¥Û∑¥∂¯ºı–°°£

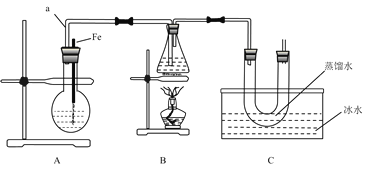
°æƒø°øƒ≥—–æø–‘—ßœ∞–°◊ȵƒÕ¨—߅˺∆¡À»ÁÕº◊∞÷√÷∆»°‰Â±Ω∫Չ““ÕÈ£∫
º∫÷™£∫““¥º‘⁄º”»»µƒÃıº˛œ¬ø…”ÎHBr∑¥”¶µ√µΩ‰Â““ÕÈ£®CH3CH2Br£©£¨∂˛’þƒ≥–©ŒÔ¿Ì–‘÷ »Áœ¬±ÌÀ˘ æ£∫
»ÐΩ‚–‘£®±æ…Ìæ˘ø…◊˜»Ðº¡£© | ∑–µ„£®°Ê£© | √Ð∂»£®g/mL£© | |
““¥º | ”ÎÀƪ•»Ð£¨“◊»Ð”⁄”–ª˙»Ðº¡ | 78.5 | 0.8 |
‰Â““ÕÈ | ƒ—»Ð”⁄ÀÆ£¨“◊»Ð”⁄”–ª˙»Ðº¡ | 38.4 | 1.4 |
«Îªÿ¥œ¬¡–Œ £∫
£®1£© B÷–∑¢…˙∑¥”¶…˙≥…ƒø±Í≤˙ŒÔµƒªØ—ß∑Ω≥Ã ΩŒ™_________°£
£®2£©∏˘æ𠵗ȃøµƒ£¨—°‘Òœ¬¡–∫œ µƒ µ—È≤Ω÷Ë£∫¢Ÿ°˙___________£®—°ÃÓ¢⁄¢€¢Ðµ»£©°£
¢Ÿ◊È◊∞∫√◊∞÷√£¨___________£®ÃÓ–¥ µ—È≤Ÿ◊˜√˚≥∆£©£ª
¢⁄Ω´A◊∞÷√÷–µƒ¥øÃ˙Àø–°–ƒœÚœ¬≤»αΩ∫Õ“∫‰ÂµƒªÏ∫œ“∫÷–£ª
¢€µ„»ºB◊∞÷√÷–µƒæ∆æ´µ∆£¨”√–°ªª∫ª∫∂‘◊∂–Œ∆øº”»»10∑÷÷”£ª
¢ÐœÚ…’∆ø÷–º”»Î“ª∂®¡ø±Ω∫Õ“∫‰Â£¨œÚ◊∂–Œ∆ø÷–º”»ÎŒÞÀÆ““¥º÷¡…‘∏þ”⁄Ω¯∆¯µºπÐø⁄¥¶£¨œÚU–ŒπÐ÷–º”»Î’Ù¡ÛÀÆ∑‚◊°πе◊£¨œÚÀÆ≤€÷–º”»Î±˘ÀÆ°£
£®3£©ºÚ ˆ µ—È÷–”√¥øÃ˙Àø¥˙ÃÊÃ˙∑€µƒ”≈µ„£∫_____°£
£®4£©±˘ÀƵƒ◊˜”√ «_______°£
£®5£©∑¥”¶Õͱœ∫Û£¨U–ŒπЃ⁄µƒœ÷œÛ «______________£ª∑÷¿Î‰Â““ÕÈ ±À˘–˵ƒ≤£¡ß“«∆˜”–_____°£