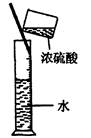
��Ŀ����
������ˮ�к��ж�������,ijУ��ѧ�о���ѧϰС���ͬѧΪ̽��������,��������ʵ��,���������ɣ�
��1��������ʹʪ��ĺ�ɫ������ɫ��ʹ����ɫ�����Ļ�ѧʽ��______
��2������ˮ�ڹ�����һ��ʱ�䣬��Һ��ɫ��dz�����йط�Ӧ�Ļ�ѧ����ʽΪ��
��
��3��ƽ�ⳣ�������˷����ϵ�Ŀ��淴Ӧ�ڸ������¶��½��еij̶ȣ�����ͬһ�����͵ķ�Ӧ��ƽ�ⳣ��Խ������Ӧ���еij̶� Խ��
Խ��
H2CO3
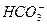 �� H�� Ka1��H2CO3��=4.45��10��7
�� H�� Ka1��H2CO3��=4.45��10��7
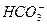

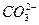 ��H�� Ka2(HCO3��)=5.61��10��11
��H�� Ka2(HCO3��)=5.61��10��11
HclO H����
H����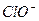 Ka(HClO)=2.95��10��8
Ka(HClO)=2.95��10��8
���������ϵ���ƽ�ⳣ������д��������������ͨ�뵽������̼������Һ����������Ӧ�����ӷ���ʽ����
��4��������ˮ��ʯ��ʯ�ķ�Ӧ����ȡ��ŨHClO��Һ�ķ���֮һ��
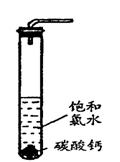
ʵ��һ�������о���
�� ���Թ��м�������Ŀ�״̼��ƣ��ټ���Լ20mL������ˮ����ַ�Ӧ��
���������ݲ�������Һdz����ɫ��ȥ��
�� ���ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ��
�� Ϊ��ȷ����Ӧ�������Һ��Ϊ���ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ���������������ɫ���壻
�������ݼ��ȣ�������Һ��������д�����ɫ���������
����⣬����ʵ���в�������ɫ�����ΪCO2��
��ش�
�� ��Ӧ�����õ���ҺƯ������ǿ��ԭ����______ ___________ ____
����������ʵ�����֪���ڵ���Һ�е����ʳ�CaCl2��HClO�⣬������_______ ��
ʵ����������о���
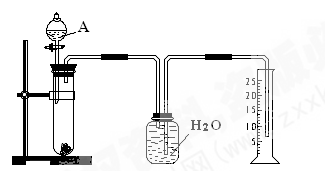
��Բ����ƿ�ײ�����һ����������ס�Ĺ�����״ ̼��ƺ�150mL������ˮ������ͼ��ʾװ��ʵ�飬�����ٲ������ݺ���������ʣ���ʯ��ʯ���Һ �棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��
�棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��

��ش�
���������1����������3��������
��1�� ��3��
��Ϊ������װ�â��ռ����������CO2���ܽ����ɵ���ʧ����ˮ������ȻΪˮ�������װ�â���иĽ�����ķ����� ��
�����ȷ������Ͳ����������
a________ _
b �����ƶ���Ͳ����Ͳ��Һ����ˮ��Һ����ƽ
c
��1��������ʹʪ��ĺ�ɫ������ɫ��ʹ����ɫ�����Ļ�ѧʽ��______
��2������ˮ�ڹ�����һ��ʱ�䣬��Һ��ɫ��dz�����йط�Ӧ�Ļ�ѧ����ʽΪ��
��
��3��ƽ�ⳣ�������˷����ϵ�Ŀ��淴Ӧ�ڸ������¶��½��еij̶ȣ�����ͬһ�����͵ķ�Ӧ��ƽ�ⳣ��Խ������Ӧ���еij̶�
 Խ��
Խ��H2CO3

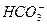 �� H�� Ka1��H2CO3��=4.45��10��7
�� H�� Ka1��H2CO3��=4.45��10��7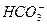

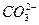 ��H�� Ka2(HCO3��)=5.61��10��11
��H�� Ka2(HCO3��)=5.61��10��11HclO
 H����
H����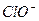 Ka(HClO)=2.95��10��8
Ka(HClO)=2.95��10��8���������ϵ���ƽ�ⳣ������д��������������ͨ�뵽������̼������Һ����������Ӧ�����ӷ���ʽ����
��4��������ˮ��ʯ��ʯ�ķ�Ӧ����ȡ��ŨHClO��Һ�ķ���֮һ��

ʵ��һ�������о���
�� ���Թ��м�������Ŀ�״̼��ƣ��ټ���Լ20mL������ˮ����ַ�Ӧ��
���������ݲ�������Һdz����ɫ��ȥ��
�� ���ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ��
�� Ϊ��ȷ����Ӧ�������Һ��Ϊ���ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ���������������ɫ���壻
�������ݼ��ȣ�������Һ��������д�����ɫ���������
����⣬����ʵ���в�������ɫ�����ΪCO2��
��ش�
�� ��Ӧ�����õ���ҺƯ������ǿ��ԭ����______ ___________ ____
����������ʵ�����֪���ڵ���Һ�е����ʳ�CaCl2��HClO�⣬������_______ ��
ʵ����������о���
��Բ����ƿ�ײ�����һ����������ס�Ĺ�����״ ̼��ƺ�150mL������ˮ������ͼ��ʾװ��ʵ�飬�����ٲ������ݺ���������ʣ���ʯ��ʯ���Һ
 �棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��
�棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��
��ش�
���������1����������3��������
��1�� ��3��
��Ϊ������װ�â��ռ����������CO2���ܽ����ɵ���ʧ����ˮ������ȻΪˮ�������װ�â���иĽ�����ķ����� ��
�����ȷ������Ͳ����������
a________ _
b �����ƶ���Ͳ����Ͳ��Һ����ˮ��Һ����ƽ
c
��1�� ��1�֣�
��1�֣�
��2�� +
+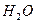
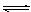
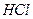 +
+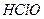 ��1�֣�
��1�֣�
2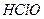
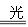 2
2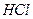 +
+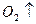 ��1�֣�
��1�֣�
��3��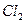 +2
+2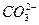 +
+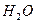
 2
2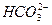 +
+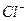 +
+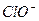 ��2�֣�
��2�֣�
��4����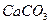 ��������ˮ�е�
��������ˮ�е�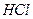 ��ʹ
��ʹ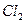 +
+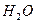
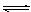
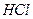 +
+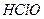 ƽ�������ƶ�
ƽ�������ƶ� 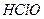 Ũ������2�֣�
Ũ������2�֣�
��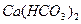 ��д����Ҳ�ԣ�2��
��д����Ҳ�ԣ�2��
�ۣ�1��Բ����ƿ ��1�֣� ��3����Ͳ��1�֣�
���ڵ���ĩ�������ӳ����ܣ�ʹ���ܵij��ڽӽ���Ͳ�ײ���2�֣�
��a��ƿ��ȴ������ ��1�֣� cƽ�ӿ̶��߶�����1�֣�
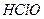 ��1�֣�
��1�֣� ��2��
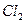 +
+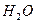
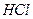 +
+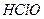 ��1�֣�
��1�֣� 2
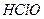
 2
2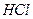 +
+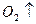 ��1�֣�
��1�֣���3��
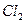 +2
+2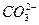 +
+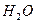
 2
2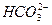 +
+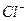 +
+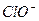 ��2�֣�
��2�֣���4����
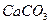 ��������ˮ�е�
��������ˮ�е�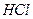 ��ʹ
��ʹ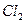 +
+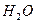
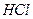 +
+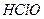 ƽ�������ƶ�
ƽ�������ƶ� 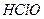 Ũ������2�֣�
Ũ������2�֣���
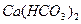 ��д����Ҳ�ԣ�2��
��д����Ҳ�ԣ�2���ۣ�1��Բ����ƿ ��1�֣� ��3����Ͳ��1�֣�
���ڵ���ĩ�������ӳ����ܣ�ʹ���ܵij��ڽӽ���Ͳ�ײ���2�֣�
��a��ƿ��ȴ������ ��1�֣� cƽ�ӿ̶��߶�����1�֣�
��

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ
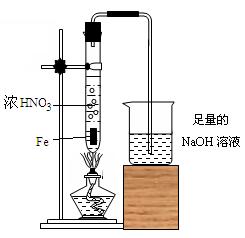
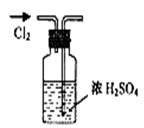
 ����ʳ����ȡŨHCl��ŨHCl����KMnO4��ȡC
����ʳ����ȡŨHCl��ŨHCl����KMnO4��ȡC l2��ѡ��
l2��ѡ�� ��ͼ��ʾװ�ã�������ʢ�ŵ��Լ������ʵ�顣
��ͼ��ʾװ�ã�������ʢ�ŵ��Լ������ʵ�顣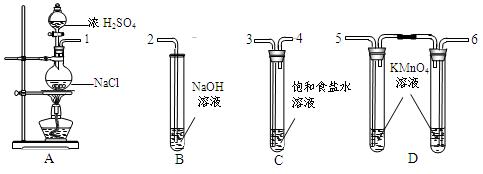
 HCl��Cl2�Ļ�ѧ��Ӧ����ʽ��
HCl��Cl2�Ļ�ѧ��Ӧ����ʽ�� _________________________________��
_________________________________�� mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣�
mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣� ����Ԥ������ͽ��ۡ�
����Ԥ������ͽ��ۡ� ____ ��Һ���μӵ��Թ�A�У���ַ�Ӧ
____ ��Һ���μӵ��Թ�A�У���ַ�Ӧ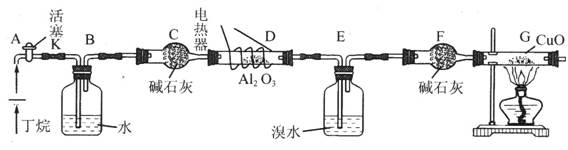 ע��CuO�ܽ���������CO2��H2O�������װ������ȥ��
ע��CuO�ܽ���������CO2��H2O�������װ������ȥ�� �������I��II�IJ������Ʒֱ��ǣ�I ,II ��
�������I��II�IJ������Ʒֱ��ǣ�I ,II ��