��Ŀ����
(16��)ij��Ͻ�����ĩ,��������������ͭ�е�һ�ֻ����֣�����������������5%���ϡ�����ƺ���ʵ��̽���û���������ĩ������ͭԪ�صĴ��ڡ�����ѡ����������Լ����ձ����Թܡ�����������Ͳ������ƿ���ιܡ�ҩ�ף�1mol/L���ᡢ2 mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣�
mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣�
[�������] ����1 �û�Ͻ�����ĩ�г�������� Ԫ�أ�
����2 �û�Ͻ�����ĩ�г�������� Ԫ�أ�
����3 �û�Ͻ�����ĩ�г��������Fe��CuԪ�أ�
[���ʵ�鷽��]���ڼ���3����Ƴ�ʵ�鷽������Ҫ�ڴ�������𣩡�
[ʵ�����]��������ʵ�鷽�������ʵ�����ز� ����Ԥ������ͽ��ۡ�
����Ԥ������ͽ��ۡ�
[��˼��д����ʵ����з�����Ӧ�����ӷ���ʽ�� ___________ ��
 mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣�
mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣�[�������] ����1 �û�Ͻ�����ĩ�г�������� Ԫ�أ�
����2 �û�Ͻ�����ĩ�г�������� Ԫ�أ�
����3 �û�Ͻ�����ĩ�г��������Fe��CuԪ�أ�
[���ʵ�鷽��]���ڼ���3����Ƴ�ʵ�鷽������Ҫ�ڴ�������𣩡�
[ʵ�����]��������ʵ�鷽�������ʵ�����ز�
 ����Ԥ������ͽ��ۡ�
����Ԥ������ͽ��ۡ�| ��� | ʵ����� | ʵ������ | ���� |
| �� | ��ҩ��ȡ������Ʒ�������Թ�A�У����õι�ȡ����  ____ ��Һ���μӵ��Թ�A�У���ַ�Ӧ ____ ��Һ���μӵ��Թ�A�У���ַ�Ӧ | �й���ʣ�࣬�������ݲ��� | �Ͻ��г��������Fe��Cu Ԫ�� |
| �� | ���Թ�A��ʣ������мӹ��� ________ ����ַ�Ӧ���ã�ȡ�ϲ���Һ���Թ�B�� | ���岿���ܽ⣬��������ų�����Һ��dz��ɫ | |
| �� | ���Թ�B�м������� _____���ٵμ�KSCN��Һ | _____ | |
| �� | ����ʣ������м���ϡ����ٵμ� ____________ ��Һ. | �����ܽ⣬����ɫ�̼�������������ܿ��ɺ���ɫ����Һ����ɫ���ټ�ij��Һ������ɫ�������� |
(��16�֣�ÿ��2��)
��������衿 ����1 ���� ����2 ͭ�������Ⱥ�˳��д��ѧʽҲ�÷֣�
��ʵ����̡�
����˼��2Al + 2OH-+ 2H2O = 2AlO2-+ 3H2��
��������衿 ����1 ���� ����2 ͭ�������Ⱥ�˳��д��ѧʽҲ�÷֣�
��ʵ����̡�
| ��� | ʵ����� | ʵ������ | ���� |
| �� | NaOH��Һ | | |
| �� | ���� | | |
| �� | ���� | ��Һ��dz��ɫ��Ϊ��ɫ���ٱ�ΪѪ��ɫ��д����Ѫ��ɫ�������֣� | |
| �� | NaOH��Һ | |
��

��ϰ��ϵ�д�
�����Ŀ
 Խ��
Խ��
 �� H�� Ka1��H2CO3��=4.45��10��7
�� H�� Ka1��H2CO3��=4.45��10��7 ��H�� Ka2(HCO3��)=5.61��10��11
��H�� Ka2(HCO3��)=5.61��10��11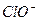 Ka(HClO)=2.95��10��8
Ka(HClO)=2.95��10��8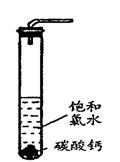
 �棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��
�棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��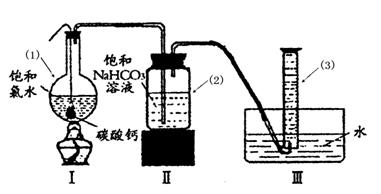
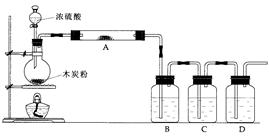
 2�����Ʊ���ijͬѧ�������һ���Ʊ�̼��Ƶķ�����������ͼΪ��
2�����Ʊ���ijͬѧ�������һ���Ʊ�̼��Ƶķ�����������ͼΪ��

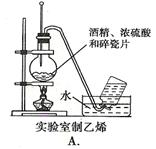
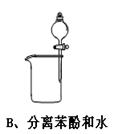
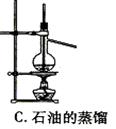
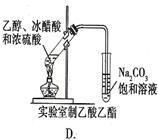

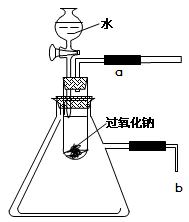
 Ŀ��
Ŀ�� ���ʹ���Ļ��Һ�м��������
���ʹ���Ļ��Һ�м��������