��Ŀ����
����Ŀ��Cr2O72-��Cr3+�Ի������м�ǿ����Ⱦ�ԣ�����Cr2O72-��Cr3+�Ĺ�ҵ��ˮ������NaOH����������ȥ��
��֪���ٳ����£�Cr3+��ȫ����(c��1.0��105 mol�� L1) ʱ����Һ��pHΪ5��NaOH����ʱCr(OH)3�ܽ�����CrO2-��Cr2O72-��ԭ����ΪCr3+��
��ش��������⣺
��1��д��Cr(OH)3����NaOH�����ӷ���ʽ______________________
��2�������£�Cr(OH)3���ܶȻ�����Ksp[Cr(OH)3]=___________��
��3�������£���50 mL 0.005 mol��L1��Cr2(SO4)3��Һ�м���0.24 mol��L1��NaOH��Һ50 mL����ַ�Ӧ����ҺpHΪ______��
��4��Ϊ�˲ⶨ��ҵ��ˮ��Na2Cr2O7��Ũ�ȣ��������²��裺
����ȡ100 mL��Һ��
������c mol��L1�ı�KMnO4������Һ�ζ�b mLһ��Ũ�ȵ�FeSO4��Һ������KMnO4��Һb mL��
����ȡb mL��Һ��������FeSO4��Һ�ζ����ﵽ�ζ��յ�ʱ������d mL FeSO4��Һ��
�ٲ������еĵζ�����Ӧѡ��_______(������ʽ��������ʽ��)�ζ��ܣ��ζ���װҺǰ�IJ�����_______��
�ڲ���������Һ��Na2Cr2O7�ĺ���Ϊ_______mol��L1��
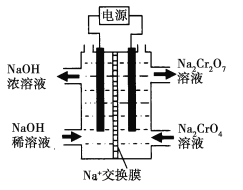
��5������ͼʾװ�ã���Ϊ���Ե缫�����Na2CrO4��Һ��ȡNa2Cr2O7��ͼ���Ҳ�缫���ӵ�Դ��______����Na2CrO4����Na2Cr2O7�����ӷ���ʽ_______________________�������缫���������2.24L�����壬��ͨ������Ĥ�������ӵ���ĿΪ___________________
 ��
��
���𰸡�Cr(OH)3+OH-![]() CrO2-+2H2O 1.0��1032 13 ��ʽ ��ϴ 5cd/6b �� 2CrO4-+2H+
CrO2-+2H2O 1.0��1032 13 ��ʽ ��ϴ 5cd/6b �� 2CrO4-+2H+![]() Cr2O72- +H2O 0.2NA��1.204��1023��
Cr2O72- +H2O 0.2NA��1.204��1023��
��������
��1��Cr��OH��3����NaOH��Һ��Ӧ����NaCrO2��
��2������Ksp[Cr��OH��3]=c��Cr3+��c3��OH-�����м��㣻
��3������pHֵ�Ķ�����м��㣻
��4�������Ը��������Һ������ǿ�����ԣ���ѡ����ʽ�ζ��ܣ��ζ���װҺǰ�IJ�������ϴ��
�ڸ���MnO4-��5Fe2+��Cr2O72-��6Fe2+����Na2Cr2O7�ĺ�����
��5������2H2O+2e��=2OH��+H2�����������Ϊ�������Ҳ�Ϊ�������������������Դ������������������ˮ�ŵ磬����������H+ʹNa2CrO4ת��ΪNa2Cr2O7��
��1���������֪��NaOH����ʱCr(OH)3�ܽ�����CrO2-����Ӧ�����ӷ���ʽΪ��Cr��OH��3+OH-![]() CrO2-+2H2O��
CrO2-+2H2O��
�ʴ�Ϊ��Cr��OH��3+OH-![]() CrO2-+2H2O��
CrO2-+2H2O��
��2�������£�Cr3+��ȫ������c��1.0��10-5molL-1��ʱpHΪ5����c��H+��=10-5mol/L��c��OH-��=10-9molL-1��c��Cr3+��=1.0��10-5molL-1����Cr��OH��3���ܶȻ�����Ksp[Cr��OH��3]=c��Cr3+��c3��OH-��=1.0��10-32mol4L-4���ʴ�Ϊ��1.0��10-32��
��3��50mL0.005mol��L1��Cr2(SO4)3��Һ�м���0.24mol��L1��NaOH��Һ50mL�����Ϻ���Һ�е�c��Cr3+��=0.005mol��L1��c��OH-��=0.12mol��L1��������Ӧ��Cr3++3OH-=Cr��OH��3����Ӧ���c��OH-��=0.12mol��L1-0.015mol��L1=0.105 mol��L1�����Գ�ַ�Ӧ����Һ��pH=14-pOH=14+lgc��OH-��=13���ʴ�Ϊ��13��
��4���ٲ���������Եı���ҺΪ���Ը��������Һ������ǿ�����ԣ���ѡ����ʽ�ζ��ܣ��ζ���װҺǰ�IJ�������ϴ���ʴ�Ϊ����ʽ����ϴ��
��cmol��L1�ı�KMnO4������Һ�ζ�bmLһ��Ũ�ȵ�FeSO4��Һ������KMnO4��ҺbmL��Mn��+7�۽�Ϊ+2�ۣ�Fe��+2����Ϊ+3�ۣ����ݵ�ʧ�����غ㣬���ڷ�Ӧ��ϵ��MnO4-��5Fe2+����ζ����õ�FeSO4��Ũ��Ϊc��Fe2+��=5cmol/L��ȡbmL��Һ��������FeSO4��Һ�ζ����ﵽ�ζ��յ�ʱ������dmLFeSO4��Һ��Cr��+6�۽�Ϊ+3�ۣ�Fe��+2����Ϊ+3�ۣ����ݵ�ʧ�����غ㣬���ڷ�Ӧ��ϵ��Cr2O72-��6Fe2+����bmL��Һ��Na2Cr2O7�ĺ���Ϊ1/6��5c��d/b=5cd/6bmol/L���ʴ�Ϊ��5cd/6b��
��5������װ��ͼ������ͼ���Ҳ�Na2CrO4ת��ΪNa2Cr2O7�����NaOHϡ��Һת��ΪNaOHŨ��Һ�������ķ�ӦΪ��2H2O+2e��=2OH��+H2�����������Ϊ�������Ҳ�Ϊ�������������������Դ������������������ˮ�ŵ磬����������H+ʹNa2CrO4ת��ΪNa2Cr2O7�����ӷ���ʽΪ��2CrO4-+2H+![]() Cr2O72-+H2O��span>�����缫���������2.24L��0.1mol������ʱ����ͨ������Ĥ��������Ϊ0.2mol�������ӵ���ĿΪ0.2NA����1.204��1023�����ʴ�Ϊ��0.2NA��1.204��1023����
Cr2O72-+H2O��span>�����缫���������2.24L��0.1mol������ʱ����ͨ������Ĥ��������Ϊ0.2mol�������ӵ���ĿΪ0.2NA����1.204��1023�����ʴ�Ϊ��0.2NA��1.204��1023����

����Ŀ������������������Ⱦ�������أ�ԭ��֮һ�ǻ�����β���к���NO��NO2��CO�����壬�������糧�ͷų�������NOx��SO2��CO2������Ҳ����ԭ�����ڶ����е�һЩ���������һ�����о���
��1���� CH4����ԭ��������������������������Ⱦ��
��֪����CH4(g) + 4NO2(g) = 4NO(g) + CO2(g) + 2H2O(g) ��H = - 574 kJ/mol
��CH4(g) + 4NO(g) = 2N2(g) + CO2(g) + 2H2O(g) ��H = - 1160 kJ/mol
��H2O(g) = H2O(l) ��H = - 44.0 kJ/mol
д�� CH4(g)�� NO2(g)��Ӧ���� N2(g)��CO2(g)�� H2O(l)���Ȼ�ѧ����ʽ�� _____________________________________________________________��
��2������β���к���CO��NO2���ж���������������װβ������װ������ʹ�ж�����ת��Ϊ�����塣4CO(g)��2NO2(g) ![]() 4CO2(g)��N2(g) ��H��-1200 kJ��mol-1
4CO2(g)��N2(g) ��H��-1200 kJ��mol-1
���ڸ÷�Ӧ���¶Ȳ�ͬ��T2��T1��������������ͬʱ������ͼ����ȷ����________________������ţ���

��3���û���̿��ԭ��Ҳ���Դ����������ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO��������ӦC(s)+2NO(g) ![]() N2(g)+CO2(g) ��H=a kJ/mol
N2(g)+CO2(g) ��H=a kJ/mol
��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ���Ũ�����£�
ʱ��/min Ũ��/(mol/L) | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 1.0 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
�ٸ���ͼ�����ݷ���T1��ʱ���÷�Ӧ��0-20min��ƽ����Ӧ����v��NO��=_____________________������÷�Ӧ��ƽ�ⳣ��K=____________________��
��30min��ֻ�ı�ijһ�����������ϱ��������жϸı������������_________������ĸ���ţ���
A��ͨ��һ������CO2 B��������ʵĴ���
C���ʵ���С��������� D��ͨ��һ������NO
E������һ�����Ļ���̿
����30min�������¶���T2�����ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ2:1:1����ﵽ��ƽ��ʱNO��ת����____________��������������������������a________0������>������<������
��4���¶�T1��T2ʱ���ֱ�0.50 mol CH4��1.2mol NO2����1 L���ܱ������з�����Ӧ��CH4(g)��2NO2(g)![]() N2(g)��CO2(g)��2H2O(g) ��H=bkJ/mol��
N2(g)��CO2(g)��2H2O(g) ��H=bkJ/mol��
����й��������±���
�¶� | ʱ��/min ���ʵ��� | 0 | 10 | 20 | 40 | 50 |
T1 | n��CH4��/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
T2 | n��CH4��/mol | 0.50 | 0.30 | 0.18 | x | 0.15 |
����˵����ȷ����__________________��
A��T1��T2����b��0
B�����¶�ΪT2����Ӧ���е�40 minʱ�� x��0.15
C���¶�ΪT2ʱ������ƽ�����������ٳ���0.50 mol CH4��1.2mol NO2�����´ﵽƽ��ʱ��n��N2��<0.70mol
D���¶�ΪT1ʱ������ʼʱ�������г���0.50 mol CH4(g)��0.50 molNO2(g)��1.0 mol N2(g)��2.0 molCO2(g)��0.50molH2O(g)����Ӧ��ʼʱ����(��)����(��)