��Ŀ����
�л����C��H��������ȼ�պ���CuO���յȷ���ʹ֮���� ��ˮ�������ǵ���������л�����̼����İٷֺ���������ij��̬�л����ڼ�������¹�������������ʹ����ȫȼ�գ���������������
��ˮ�������ǵ���������л�����̼����İٷֺ���������ij��̬�л����ڼ�������¹�������������ʹ����ȫȼ�գ���������������
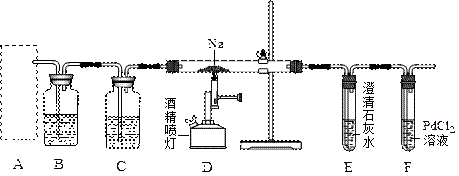
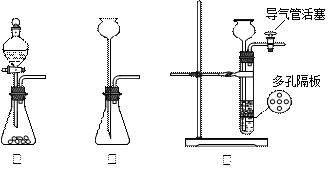
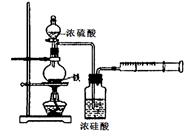
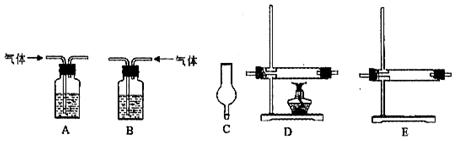
��1��Ϊȷ��ij�����л�������ʽ��ʵ��ʽ������������������Ҫ��������________�֣�������________������Ҫһǰһ�������ε���________������������ţ�������ǰ��Ϊ________������Ϊ________����Ϊ��ɲⶨ��ȱ�ٵ�һ����Ҫ����������________��
��2��Ϊȷ�������л������������京�������ʵ��������________��
��3��Ϊȷ�������ʽ����֪�������е�
A. �ۡ��е㡡���� B. ���¡���ѹ�������ܶ� ��
C. �̶��л��������� D. ��Ҫ�Ļ�ѧ����
 ��ˮ�������ǵ���������л�����̼����İٷֺ���������ij��̬�л����ڼ�������¹�������������ʹ����ȫȼ�գ���������������
��ˮ�������ǵ���������л�����̼����İٷֺ���������ij��̬�л����ڼ�������¹�������������ʹ����ȫȼ�գ�����������������1��Ϊȷ��ij�����л�������ʽ��ʵ��ʽ������������������Ҫ��������________�֣�������________������Ҫһǰһ�������ε���________������������ţ�������ǰ��Ϊ________������Ϊ________����Ϊ��ɲⶨ��ȱ�ٵ�һ����Ҫ����������________��

��2��Ϊȷ�������л������������京�������ʵ��������________��
��3��Ϊȷ�������ʽ����֪�������е�
A. �ۡ��е㡡���� B. ���¡���ѹ�������ܶ� ��
C. �̶��л��������� D. ��Ҫ�Ļ�ѧ����
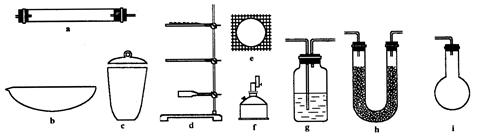
��1��4��a��d��f��h��h����
 ������
������ ����ƽ
����ƽ��2������������̼Ԫ����������Ԫ������Ϊ��
����������̼Ԫ����������Ԫ������Ϊ���ĺ���������
��3��B
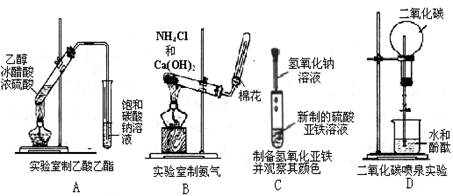
�����л���ͻ�ѧʵ������ϡ��ⶨ�л����ʵ��ʽ����Ҫ�ⶨ�л��ﱾ���������� ��ˮ���������ⶨˮ����������Ũ�������ճ��أ��ⶨ
��ˮ���������ⶨˮ����������Ũ�������ճ��أ��ⶨ ���������ü�ʯ�����ղ����ء�
���������ü�ʯ�����ղ����ء�
 ��ˮ���������ⶨˮ����������Ũ�������ճ��أ��ⶨ
��ˮ���������ⶨˮ����������Ũ�������ճ��أ��ⶨ ���������ü�ʯ�����ղ����ء�
���������ü�ʯ�����ղ����ء�
��ϰ��ϵ�д�
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�����Ŀ

 ������(C6H12O6)
������(C6H12O6)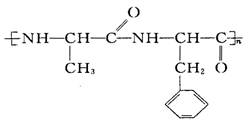 ˮ�����ķ��ӽṹ��ʽ�����Ʒֱ��� __________________ ��______________________ ��
ˮ�����ķ��ӽṹ��ʽ�����Ʒֱ��� __________________ ��______________________ ��