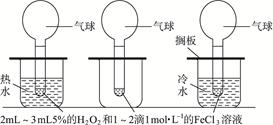
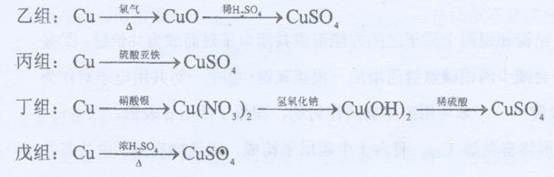
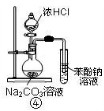
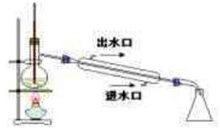
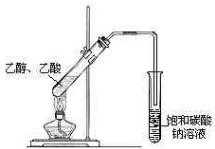
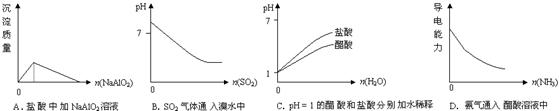
��Ŀ����
2001��6��21�գ����ϵ�֣�ݡ�����������������ʹ�����������÷�����з�ʽ�������µ�����ȼ�ϡ��������Ҵ����͡��Ҵ��������ƾ������������ס�С�������Ϊԭ�Ͼ����͡�������Ƴɵġ��Ҵ���һ����ˮ���ټ����������ͺ��γɱ���ȼ���Ҵ����������Ҵ����;��ǰѱ���ȼ���Ҵ������Ͱ�һ�����������γɵij���ȼ�ϡ�����й�֪ʶ������������⣺
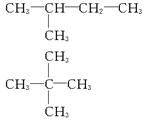
(1)�Ҵ��Ľṹ��ʽΪ_____________����������ʯ�ͷ������õĵͷе�������������е�̼ԭ����һ����C5��C11��Χ�ڣ������飬�����ʽΪ__________________���ṹ��ʽ����ͬ���칹��ֱ�Ϊ_____________��_____________��_____________��
(2)�Ҵ����ɺ����ۡ�(C6H10O5)n����ũ��Ʒ�������ס�С������Ⱦ����͡�������á���д���ɵ������Ҵ��Ļ�ѧ����ʽ��
�ٵ���+ˮ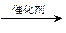 ������(C6H12O6)
������(C6H12O6)
��������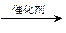 �Ҵ�
�Ҵ�
(3)���ۿ�����ɫֲ�ᆳ������õ�һϵ�����ﻯѧ��Ӧ�õ�����ˮ�Ͷ�����̼������������������ǣ��������������ɵ��ۡ����й�����õij�����_____________����������������������ǵĻ�ѧ����ʽ��
__________________________________________________________��
(4)�Ҵ����ȼ�յIJ���Ϊ__________________��__________________��
(5)�����Ҵ����ͳ�Ϊ����ȼ�ϣ���ԭ����__________________________________________��
(1)�Ҵ��Ľṹ��ʽΪ_____________����������ʯ�ͷ������õĵͷе�������������е�̼ԭ����һ����C5��C11��Χ�ڣ������飬�����ʽΪ__________________���ṹ��ʽ����ͬ���칹��ֱ�Ϊ_____________��_____________��_____________��
(2)�Ҵ����ɺ����ۡ�(C6H10O5)n����ũ��Ʒ�������ס�С������Ⱦ����͡�������á���д���ɵ������Ҵ��Ļ�ѧ����ʽ��
�ٵ���+ˮ
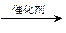 ������(C6H12O6)
������(C6H12O6)��������
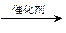 �Ҵ�
�Ҵ�(3)���ۿ�����ɫֲ�ᆳ������õ�һϵ�����ﻯѧ��Ӧ�õ�����ˮ�Ͷ�����̼������������������ǣ��������������ɵ��ۡ����й�����õij�����_____________����������������������ǵĻ�ѧ����ʽ��
__________________________________________________________��
(4)�Ҵ����ȼ�յIJ���Ϊ__________________��__________________��
(5)�����Ҵ����ͳ�Ϊ����ȼ�ϣ���ԭ����__________________________________________��
��1��C2H5OH C5H12 CH3��CH2��CH2��CH2��CH3
��2����(C6H10O5)n+nH2O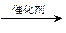 nC6H12O6
nC6H12O6
��C6H12O6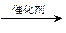 2C2H5OH+2CO2
2C2H5OH+2CO2
��3��Ҷ���� 6CO2+6H2O C6H12O6+6O2
C6H12O6+6O2
��4��CO2 H2O
��5������Ч��������β�����������ش�����Ⱦ�����ƻ�������

��2����(C6H10O5)n+nH2O
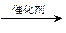 nC6H12O6
nC6H12O6��C6H12O6
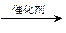 2C2H5OH+2CO2
2C2H5OH+2CO2��3��Ҷ���� 6CO2+6H2O
 C6H12O6+6O2
C6H12O6+6O2��4��CO2 H2O
��5������Ч��������β�����������ش�����Ⱦ�����ƻ�������
�����ǻ�ѧ���������ϵ���Ŀ���Ҵ�������ȼ�գ�����CO2��ˮ������Ի��������Ⱦ��

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ
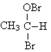
 ��һ�ֹ����Լ������Լ�������Ϊ_________��B���ܲ���������������������ԭ
��һ�ֹ����Լ������Լ�������Ϊ_________��B���ܲ���������������������ԭ ��(��ϻ�ѧ����ʽ�ش�)_ __��
��(��ϻ�ѧ����ʽ�ش�)_ __��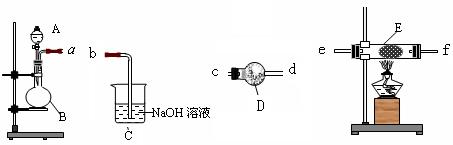

 4NO2(g)����H>0���±�Ϊ��Ӧ��ij�¶��µIJ���ʵ������:
4NO2(g)����H>0���±�Ϊ��Ӧ��ij�¶��µIJ���ʵ������: ��ˮ�������ǵ���������л�����̼����İٷֺ���������ij��̬�л����ڼ�������¹�������������ʹ����ȫȼ�գ���������������
��ˮ�������ǵ���������л�����̼����İٷֺ���������ij��̬�л����ڼ�������¹�������������ʹ����ȫȼ�գ���������������