��Ŀ����
 ��1�������ԭ�Ӻ�����������Dz�ͬ�ģ������ܲ�������n��ԽС�����ܲ�����
��1�������ԭ�Ӻ�����������Dz�ͬ�ģ������ܲ�������n��ԽС�����ܲ�����Խ��
Խ��
����ͬһ�ܲ��У����ܼ���������ns��np��nd��nf�Ĵ�������
����
����2��ԭ�ӵĵ����Ų���ѭ����ԭ����ʹ����ԭ�ӵ������������״̬���������������ԭ�ӽ���
��̬
��̬
ԭ�ӣ�����Щԭ������
����
���������ԾǨ���ϸ��ܼ���ͱ�ɼ���̬����3����ͼ�����ڱ��ж���������Ԫ�ص�һ���֣����е縺������Ԫ����
B
B
����һ��������С��Ԫ����C��D
C��D
����������1���ܲ�������n��ԽС���������Խ��������Խ�ͣ�
��2���������������ԭ�ӽ�����̬ԭ�ӣ����������ɴﵽ����̬��
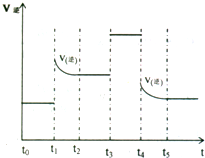
��3�����ݵ縺�Ե�һ�����������ڱ��еı仯�����жϣ�ע���һ�����ܵķ�������
��2���������������ԭ�ӽ�����̬ԭ�ӣ����������ɴﵽ����̬��
��3�����ݵ縺�Ե�һ�����������ڱ��еı仯�����жϣ�ע���һ�����ܵķ�������
����⣺��1���ܲ�������n��ԽС���������Խ��������Խ�ͣ���ͬһ�ܲ��У����ܼ���������ns��np��nd��nf�Ĵ���������ʴ�Ϊ��Խ�ͣ�������
��2���������������ԭ�ӽ�����̬ԭ�ӣ�����̬�����ϸߣ���̬����ԾǨ������̬����Ҫ�����������ʴ�Ϊ����̬�����գ�
��3��ͬ���ڣ������ң�Ԫ�صĵ縺��������ͬ���壺���ϵ��£�Ԫ�صĵ縺����С��B�ĵ縺�����һ�����ܵı仯��Ԫ��ԭ�ӵĺ�������Ų��йأ�ͨ������£���ԭ�Ӻ���ĵ����Ų���������ȵĹ�����γ�ȫ�գ�������ȫ���Ľṹʱ��ԭ�ӵ������ϵͣ�ԭ�ӽ��ȶ������ԭ�ӱȽ���ʧȥ���ӣ��ʵ�һ�����ܽϴ�N�ĵ�һ�����ܴ���O������Ԫ�ص�����û��ȷ�������һ��������С��Ԫ�ؿ���ΪC��D��
�ʴ�Ϊ��B��C��D��
��2���������������ԭ�ӽ�����̬ԭ�ӣ�����̬�����ϸߣ���̬����ԾǨ������̬����Ҫ�����������ʴ�Ϊ����̬�����գ�
��3��ͬ���ڣ������ң�Ԫ�صĵ縺��������ͬ���壺���ϵ��£�Ԫ�صĵ縺����С��B�ĵ縺�����һ�����ܵı仯��Ԫ��ԭ�ӵĺ�������Ų��йأ�ͨ������£���ԭ�Ӻ���ĵ����Ų���������ȵĹ�����γ�ȫ�գ�������ȫ���Ľṹʱ��ԭ�ӵ������ϵͣ�ԭ�ӽ��ȶ������ԭ�ӱȽ���ʧȥ���ӣ��ʵ�һ�����ܽϴ�N�ĵ�һ�����ܴ���O������Ԫ�ص�����û��ȷ�������һ��������С��Ԫ�ؿ���ΪC��D��
�ʴ�Ϊ��B��C��D��
���������⿼��ԭ�Ӻ�����ӵ��Ų����ɣ���Ŀ�ѶȲ�����ע����յ����Ų��ص㣬�����ǵ�һ�����ܵı仯���ɣ�Ϊ�״��㣮

��ϰ��ϵ�д�
 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�����Ŀ
 ��2009?����ģ�⣩A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������A��Eͬ���壬AԪ�ص�ԭ�Ӱ뾶��С��BԪ��ԭ�ӵ��������������ڲ��������2����CԪ��ԭ�ӵĵ��Ӳ���Ϊn������������Ϊ2n+1��A��B��C��Eÿ��Ԫ�ض�����DԪ��������ֻ��������ϵij���������ش��������⣺
��2009?����ģ�⣩A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������A��Eͬ���壬AԪ�ص�ԭ�Ӱ뾶��С��BԪ��ԭ�ӵ��������������ڲ��������2����CԪ��ԭ�ӵĵ��Ӳ���Ϊn������������Ϊ2n+1��A��B��C��Eÿ��Ԫ�ض�����DԪ��������ֻ��������ϵij���������ش��������⣺