��Ŀ����
�����£���Ũ�Ⱦ�Ϊ1 mol��L��1������4����Һ��
��H2SO4��Һ ��NaHCO3��Һ ��NH4Cl��Һ ��NaOH��Һ
��1����4����ҺpH�ɴ�С��˳���� ��������ˮ�����H��Ũ����С���� ����������ţ�
��2�����и�����Ũ���ɴ�С��˳���� ��NaHCO3��ˮ��ƽ�ⳣ��Kh�� mol��L��1������֪̼��ĵ��볣��K1��4��10��7��K2��5.6��10��11��
��3�������ͨ��������������ʱ![]() ��ֵ �����������С�����䡱����
��ֵ �����������С�����䡱����
��4�������ۺܻ͢�Ϻ���Һǡ�ó����ԣ�����ǰ�۵���� �ܵ����������ڡ�����С�ڡ����ڡ�֮һ����
(1)��2�֣� �ܢڢۢ� ��
(2)��4�֣�c(Na+) > c(HCO3��) > c(CO32��) > c(OH��) > c(H+) 2.5��10��8
(3)��1�֣���С
(4)��1�֣�����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
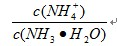 ��ֵ
��ֵ