题目内容
常温下,有浓度均为1mol?L-1的下列4种溶液:①H2SO4溶液②NaHCO3溶液③NH4Cl溶液④NaOH溶液(1)这4种溶液pH由大到小的顺序是
(2)②中各离子浓度由大到小的顺序是
(3)向③中通入少量氨气,此时
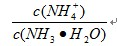 的值
的值(4)若将③和④混合后溶液恰好呈中性,则混合前③的体积
分析:(1)根据溶液的酸碱性判断溶液的pH大小,酸和碱溶液抑制了水的电离,盐溶液促进了水的电离,酸或者碱溶液中氢离子、氢氧根离子浓度越大,水的电离程度越小,据此进行解答;
(2)根据碳酸氢钠溶液中离子浓度大小进行比较;根据Kh=
=
=
计算出NaHCO3的水解平衡常数Kh;
(3)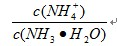 =
=
,通入氨气后,溶液中氢氧根离子浓度增大,氢离子浓度减小,该比值减小;
(4)先比较等体积混合后溶液的酸碱性,然后判断溶液显示中性时二者体积关系.
(2)根据碳酸氢钠溶液中离子浓度大小进行比较;根据Kh=
| c(HCO3-)?c(OH-) |
| c(CO32-) |
| c(HCO3-)?c(OH-)?c(H+) |
| c(CO32-)?c(H+) |
| KW |
| K2 |
(3)
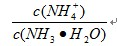 =
=| c(H+) |
| K(铵离子水解平衡常数) |
(4)先比较等体积混合后溶液的酸碱性,然后判断溶液显示中性时二者体积关系.
解答:(1)相同浓度的溶液中,①H2SO4溶液为酸性溶液,②NaHCO3溶液中碳酸根离子部分水解,溶液显示弱碱性,③NH4Cl溶液中铵离子部分水解,溶液显示弱酸性,④NaOH溶液为强碱溶液,所以这4种溶液pH由大到小的顺序为:④②③①;②③为含有弱酸根或者弱碱根离子的盐溶液,促进了水的电离,而①④分别为酸和碱溶液,抑制了水的电离,其中①电离的氢离子浓度大于④电离的氢氧根离子,所以①中水的电离程度最小,
故答案为:④②③①;①;
(2)碳酸氢钠溶液中,碳酸氢根离子水解,溶液显示碱性,碳酸氢钠溶液中离子浓度大小关系为:c(Na+)>c(HCO3-)>c(CO32-)>c(OH-)>c(H+);Kh=
=
=
=
mol/L=2.5×10-8 mol/L,
故答案为:c(Na+)>c(HCO3-)>c(CO32-)>c(OH-)>c(H+);2.5×10-8;
(3)设铵离子的水解常数为K,K=
,则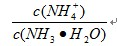 =
=
,该比值与溶液中氢离子浓度变化有关,当加入少量氨气后,溶液中氢氧根离子浓度增大,氢离子浓度减小,所以此比值减小,
故答案为:减小;
(4)若氯化铵与氢氧化钠溶液等浓度等体积混合,二者恰好反应生成氯化钠和氨水,溶液显示碱性,若要使溶液显示中性,则氯化铵的体积应该大些或者氢氧化钠溶液体积小些,即氯化铵溶液体积大于氢氧化钠溶液体积,
故答案为:大于.
故答案为:④②③①;①;
(2)碳酸氢钠溶液中,碳酸氢根离子水解,溶液显示碱性,碳酸氢钠溶液中离子浓度大小关系为:c(Na+)>c(HCO3-)>c(CO32-)>c(OH-)>c(H+);Kh=
| c(HCO3-)?c(OH-) |
| c(CO32-) |
| c(HCO3-)?c(OH-)?c(H+) |
| c(CO32-)?c(H+) |
| KW |
| K2 |
| 10-14 |
| 5.6×10-11 |
故答案为:c(Na+)>c(HCO3-)>c(CO32-)>c(OH-)>c(H+);2.5×10-8;
(3)设铵离子的水解常数为K,K=
| c(NH4+)c(OH-) |
| c(NH3?H2O) |
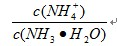 =
=| c(H+) |
| K(铵离子水解平衡常数) |
故答案为:减小;
(4)若氯化铵与氢氧化钠溶液等浓度等体积混合,二者恰好反应生成氯化钠和氨水,溶液显示碱性,若要使溶液显示中性,则氯化铵的体积应该大些或者氢氧化钠溶液体积小些,即氯化铵溶液体积大于氢氧化钠溶液体积,
故答案为:大于.
点评:本题考查了水的电离、弱电解质的电离平衡、盐的水解原理、离子浓度大小比较等知识,题目难度中等,注意明确溶液酸碱性与溶液pH的关系及计算方法,掌握影响水的电离、盐的水解的因素,能够利用电荷守恒、物料守恒等比较溶液中离子浓度大小.

练习册系列答案
 状元坊全程突破导练测系列答案
状元坊全程突破导练测系列答案
相关题目