��Ŀ����
��10�֣�ij�о���ѧϰС���о�HNO3�������ԣ����������ʵ�飺��ʢ�������Ƶ�FeSO4��Һ���Թ��е���2��KSCN��Һ���۲�����Ȼ���ټ���ŨHNO3����Һ����ɫ��죬���ǽ���ɫ��Һ����һ���������Һ�ɺ�ɫ���ٱ�Ϊ��ɫ������������ɫ���壬��һ������������ͬѧ�ǵĺ���������֪���������ǶԴ����������̽����ʵ�顣
��1����ͬѧ��Ϊ����Һ�е�Fe2+�ĸ�����ɵģ���Ҿ������Է�������Ϊ�����ų�Fe2+�ĸ��ţ����������������������� �� ������ ������������
��2����ͬѧ��Ϊ��ɫ��ʧ��˵��Fe(SCN)3���ƻ�������ɫNO2˵����ijЩ������HNO3������������ԭ��Ӧ���Ʋ������KSCN��HNO3���á�����C��S��N��ԭ�ӽṹ���ۼ������֪ʶ�ƶ�SCN-�ĽṹʽΪ ��
��3��������ͬѧ�ģ��۵㣬�����ʵ�鷽��1����ŨHNO3����μ���KSCN��Һ��ʵ�鿪ʼʱ����������һ��ʱ�����Һ�������ɫ�����ɫ ��ͻȻ���ҷ�Ӧ�����������ݣ��ų�����ɫ���壬����Һ��ɫ��ʧ��Ϊdz��ɫ����Һ�¶����ߣ���������KSCN��Һ��Ϊdz��ɫ������Ϊ��ɫ��������������ͨ�������Ba(OH)2��Һ���������ǣ���ʣ��һ�ַǼ������壻��Ӧ�����Һ�м���BaCl2��Һ������ɫ������(�˹�������Һ��ɫ�仯����ϸ��)����д����ŨHNO3�е���KSCN���ӵķ���ʽ�� ��
��ͻȻ���ҷ�Ӧ�����������ݣ��ų�����ɫ���壬����Һ��ɫ��ʧ��Ϊdz��ɫ����Һ�¶����ߣ���������KSCN��Һ��Ϊdz��ɫ������Ϊ��ɫ��������������ͨ�������Ba(OH)2��Һ���������ǣ���ʣ��һ�ַǼ������壻��Ӧ�����Һ�м���BaCl2��Һ������ɫ������(�˹�������Һ��ɫ�仯����ϸ��)����д����ŨHNO3�е���KSCN���ӵķ���ʽ�� ��
��4����ͬѧ��ΪSCN-������ ���ɽ�һ��̽��������˷���2����Fe��SCN��3�зֱ�μӹ�������ˮ����ˮ����Һ�ĺ�ɫ����ʧ��Ϊ��ɫ������������ĵ�ˮʱ��Һ����ɫ�������䡣��ͬѧ�������ͼ�� ��
��5��ͨ������̽������֪��SCN-��Ӽ���Fe2+ʱӦע�� ��
��1����ͬѧ��Ϊ����Һ�е�Fe2+�ĸ�����ɵģ���Ҿ������Է�������Ϊ�����ų�Fe2+�ĸ��ţ����������������������� �� ������ ������������
��2����ͬѧ��Ϊ��ɫ��ʧ��˵��Fe(SCN)3���ƻ�������ɫNO2˵����ijЩ������HNO3������������ԭ��Ӧ���Ʋ������KSCN��HNO3���á�����C��S��N��ԭ�ӽṹ���ۼ������֪ʶ�ƶ�SCN-�ĽṹʽΪ ��
��3��������ͬѧ�ģ��۵㣬�����ʵ�鷽��1����ŨHNO3����μ���KSCN��Һ��ʵ�鿪ʼʱ����������һ��ʱ�����Һ�������ɫ�����ɫ
 ��ͻȻ���ҷ�Ӧ�����������ݣ��ų�����ɫ���壬����Һ��ɫ��ʧ��Ϊdz��ɫ����Һ�¶����ߣ���������KSCN��Һ��Ϊdz��ɫ������Ϊ��ɫ��������������ͨ�������Ba(OH)2��Һ���������ǣ���ʣ��һ�ַǼ������壻��Ӧ�����Һ�м���BaCl2��Һ������ɫ������(�˹�������Һ��ɫ�仯����ϸ��)����д����ŨHNO3�е���KSCN���ӵķ���ʽ�� ��
��ͻȻ���ҷ�Ӧ�����������ݣ��ų�����ɫ���壬����Һ��ɫ��ʧ��Ϊdz��ɫ����Һ�¶����ߣ���������KSCN��Һ��Ϊdz��ɫ������Ϊ��ɫ��������������ͨ�������Ba(OH)2��Һ���������ǣ���ʣ��һ�ַǼ������壻��Ӧ�����Һ�м���BaCl2��Һ������ɫ������(�˹�������Һ��ɫ�仯����ϸ��)����д����ŨHNO3�е���KSCN���ӵķ���ʽ�� ����4����ͬѧ��ΪSCN-������ ���ɽ�һ��̽��������˷���2����Fe��SCN��3�зֱ�μӹ�������ˮ����ˮ����Һ�ĺ�ɫ����ʧ��Ϊ��ɫ������������ĵ�ˮʱ��Һ����ɫ�������䡣��ͬѧ�������ͼ�� ��
��5��ͨ������̽������֪��SCN-��Ӽ���Fe2+ʱӦע�� ��

��

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
�����Ŀ
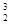 He���ֲ��������̽�⡣
He���ֲ��������̽�⡣ ԭ����ȫ����NO����Ӧ�����Һ�м���һ������NaOH��Һ��ǡ��ʹ��Ԫ����ȫת��Ϊ���������ˣ�������ϴ�ӡ�������ա����أ��ù���a�ˣ�����Һ���ɡ����ա����أ��ù���b�ˡ�
ԭ����ȫ����NO����Ӧ�����Һ�м���һ������NaOH��Һ��ǡ��ʹ��Ԫ����ȫת��Ϊ���������ˣ�������ϴ�ӡ�������ա����أ��ù���a�ˣ�����Һ���ɡ����ա����أ��ù���b�ˡ� �����У���˵��ԭ�������У���д����Ӧ�����ӷ���ʽ�� ��
�����У���˵��ԭ�������У���д����Ӧ�����ӷ���ʽ�� ��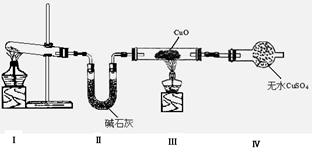
 �Ļ�ѧ����ʽΪ ��
�Ļ�ѧ����ʽΪ �� ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ��
ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ�� Ӧ�Ļ�ѧ����ʽ
Ӧ�Ļ�ѧ����ʽ  ��
�� 
 ���װ�������Ժ��Ƚ�C����˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ơ�����ʵ���������£���˿�������ֺ��ȣ�F��ͭƬ���ܽ⡣
���װ�������Ժ��Ƚ�C����˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ơ�����ʵ���������£���˿�������ֺ��ȣ�F��ͭƬ���ܽ⡣