��Ŀ����
����Ŀ��������ˮ�е�Ԫ�������ˮ�帻Ӫ��������Ҫԭ����ij��ˮ��NH4Cl����Ϊ180 mg/L��
(1)д��NH4Cl�ĵ���ʽ_________��
(2)д������ͬ���ڣ���2��δ�ɶԵ��ӵ�ԭ�ӵĵ����Ų�ʽ��_______��________
(3)Ϊ��ȥ��ˮ�е�NH4+����103 L����ˮ�м���0.1 mol/L NaOH��Һ����������ҪNaOH ��Һ�����Ϊ_________L(������������λС��)��
(4)��ij��ˮ��ͬʱ����NH4����NO3-ʱ���������з�����ȥ��������������ˮ�м�����м��NO3-ת��ΪNH4�����ٳ�ȥ������ƽ�������ӷ���ʽ���������ת�Ƶķ������Ŀ��___Fe +___NO3- +___H+=___Fe2+ +___NH4+ +___H2O��____________��
���𰸡�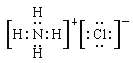 1s22s22p63s23p2 1s22s22p63s23p4 33.64 4 1 10 4 1 3
1s22s22p63s23p2 1s22s22p63s23p4 33.64 4 1 10 4 1 3 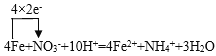
��������
(1) NH4Cl�����ӻ������NH4+��Cl-ͨ�����Ӽ����ɣ�������ӻ�����ı�ʾ������д�����ʽ��
(2)����Clԭ�Ӻ�������Ų�ʽȷ����������Ӳ������еĹ����Ŀ�����ÿһ�������Ų�2�����ӣ�ȷ������ͬһ���ڣ���2��δ�ɶԵ��ӵ�ԭ�ӵĵ����Ų�ʽ��
(3)����NH4Cl��NaOH��Һ��Ӧʱ���ߵ����ʵ����ı���1��1��������ˮ��NH4Cl����Ϊ180 mg/L������c(NH4Cl)�����n=c��V���㣻
(4)���ݵ����غ㡢����غ㡢ԭ���غ���ƽ����ʽ��
(1) NH4Cl�����ӻ������NH4+��Cl-ͨ�����Ӽ����ɣ������ʽΪ��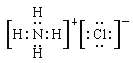 ��
��
(2)Clԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p5����������Ӳ�3p�ܼ�����3�����������ԭ�Ӻ���������Ǿ����ܳɵ����У���������������ͬ��ͬһ�������������2������������ͬһ���ڣ���2��δ�ɶԵ��ӵ�ԭ�ӵĵ����Ų�ʽ�ֱ�Ϊ1s22s22p63s23p2��1s22s22p63s23p4��������Ԫ�طֱ���Si��S��
(3)��ˮ��NH4Cl����Ϊ180 mg/L����c(NH4Cl)=![]() =
=![]() mol/L��103 L����ˮ���к���NH4Cl�����ʵ���Ϊn(NH4Cl)=
mol/L��103 L����ˮ���к���NH4Cl�����ʵ���Ϊn(NH4Cl)=![]() mol/L��103 L=3.364 mol�����ݷ�Ӧ��NH4Cl+NaOH=NaCl+NH3��+H2O����֪n(NaOH)=n(NH4Cl)=3.364 mol������NaOH��ҺŨ��Ϊ0.1 mol/L������������ҪNaOH ��Һ�����V(NaOH)=
mol/L��103 L=3.364 mol�����ݷ�Ӧ��NH4Cl+NaOH=NaCl+NH3��+H2O����֪n(NaOH)=n(NH4Cl)=3.364 mol������NaOH��ҺŨ��Ϊ0.1 mol/L������������ҪNaOH ��Һ�����V(NaOH)=![]() =33.64 L��
=33.64 L��
(4)�ڷ�Ӧ��___Fe +___NO3- +___H+=___Fe2+ +___NH4+ +___H2O�У�FeԪ�ػ��ϼ���0��+2�ۣ�����2�ۣ�NԪ�ػ��ϼ���NO3-��NH4+������8�ۣ����ϼ�������С��������8������Fe��Fe2+ϵ����4��NO3-��NH4+ϵ����1��Ȼ����ݷ�Ӧǰ�����غ㣬��֪H+��ϵ����10��������ԭ���غ㣬�ɵ�H2O��ϵ����3������ƽ��÷�Ӧ����ʽΪ��
4Fe+NO3-+10H+=4Fe2++NH4++3H2O���õ����ŷ���ʾΪ��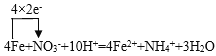 ��
��

 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д� ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�