��Ŀ����
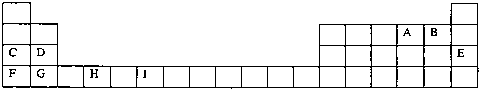
���Ϊ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�

��ش��������⣺
��1����������d����Ԫ����
��2��д��Ԫ�آ�Ļ�̬ԭ�ӵĵ����Ų�ʽ
��3��ijԪ�ص����������Ų�ʽΪnsnnpn+1����Ԫ��ԭ�ӵĺ����������ӵijɶԵ���Ϊ
��4����3����8��Ԫ�ذ������۵�ߵ͵�˳����ͼ��
������š�8������
���е縺��������

��ش��������⣺
��1����������d����Ԫ����
��
��
�����ţ�����2��д��Ԫ�آ�Ļ�̬ԭ�ӵĵ����Ų�ʽ
1S22S22P63S23P63d64S2
1S22S22P63S23P63d64S2
����3��ijԪ�ص����������Ų�ʽΪnsnnpn+1����Ԫ��ԭ�ӵĺ����������ӵijɶԵ���Ϊ
1
1
�ԣ���4����3����8��Ԫ�ذ������۵�ߵ͵�˳����ͼ��
������š�8������
Si
Si
����Ԫ�ط��ţ������е縺��������
Cl
Cl
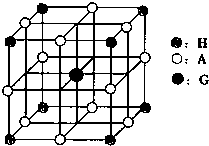
����Ԫ�ط��ţ�����������1��d��Ԫ�ذ�����B-��BԪ�أ�����Ԫ�أ�Ϊ��3�е�10��Ԫ�أ���ϵԪ�ء��ϵԪ�س��⣩��
��2����Ԫ�������ڱ��е����ʿ�֪����ΪFeԪ�أ�����26��Ԫ�أ����ݺ�������Ų�������д��̬ԭ�ӵĵ����Ų�ʽ��
��3��s�ܼ��������2�����ӣ�ͬһ�ܲ���s�ܼ������������p�ܼ�����n=2����Ԫ�ص����������Ų�ʽΪ2s2np3���ݴ˽��
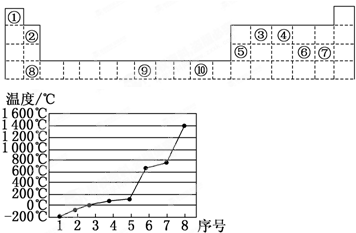
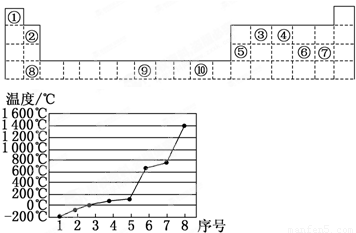
��4����3����8��Ԫ����Si��ԭ�Ӿ��壬���ʵ��۵���ߣ�
ͬ����������ҵ縺����ǿ��ϡ��������⣩��
��2����Ԫ�������ڱ��е����ʿ�֪����ΪFeԪ�أ�����26��Ԫ�أ����ݺ�������Ų�������д��̬ԭ�ӵĵ����Ų�ʽ��
��3��s�ܼ��������2�����ӣ�ͬһ�ܲ���s�ܼ������������p�ܼ�����n=2����Ԫ�ص����������Ų�ʽΪ2s2np3���ݴ˽��
��4����3����8��Ԫ����Si��ԭ�Ӿ��壬���ʵ��۵���ߣ�
ͬ����������ҵ縺����ǿ��ϡ��������⣩��
����⣺��1��d��Ԫ�ذ�����B-��BԪ�أ�����Ԫ�أ�Ϊ��3�е�10��Ԫ�أ���ϵԪ�ء��ϵԪ�س��⣩����Ԫ�������ڱ���λ�ÿ�֪���ᴦ�ڵ�8�У�Ϊ�ڢ���Ԫ�أ�����d����Ԫ�أ�
�ʴ�Ϊ���
��2����Ԫ�������ڱ��е����ʿ�֪����ΪFeԪ�أ�����26��Ԫ�أ���̬ԭ�ӵĵ����Ų�ʽΪ1S22S22P63S23P63d64S2��
�ʴ�Ϊ��1S22S22P63S23P63d64S2��
��3��s�ܼ��������2�����ӣ�ͬһ�ܲ���s�ܼ������������p�ܼ�����n=2����Ԫ�ص����������Ų�ʽΪ2s2np3��s�ܼ�Ϊ�ɶԵ��ӣ�p�ܼ�3�����Ӹ�ռ��1��������ʸ�Ԫ��ԭ�ӵĺ����������ӵijɶԵ���Ϊ1�ԣ��ʴ�Ϊ��1��
��4����3����8��Ԫ����Si��ԭ�Ӿ��壬���ʵ��۵���ߣ�ͬ����������ҵ縺����ǿ��ϡ��������⣩����ClԪ�صĵ縺����ǿ���ʴ�Ϊ��Si��Cl��
�ʴ�Ϊ���
��2����Ԫ�������ڱ��е����ʿ�֪����ΪFeԪ�أ�����26��Ԫ�أ���̬ԭ�ӵĵ����Ų�ʽΪ1S22S22P63S23P63d64S2��
�ʴ�Ϊ��1S22S22P63S23P63d64S2��
��3��s�ܼ��������2�����ӣ�ͬһ�ܲ���s�ܼ������������p�ܼ�����n=2����Ԫ�ص����������Ų�ʽΪ2s2np3��s�ܼ�Ϊ�ɶԵ��ӣ�p�ܼ�3�����Ӹ�ռ��1��������ʸ�Ԫ��ԭ�ӵĺ����������ӵijɶԵ���Ϊ1�ԣ��ʴ�Ϊ��1��
��4����3����8��Ԫ����Si��ԭ�Ӿ��壬���ʵ��۵���ߣ�ͬ����������ҵ縺����ǿ��ϡ��������⣩����ClԪ�صĵ縺����ǿ���ʴ�Ϊ��Si��Cl��
���������⿼��Ԫ�����ڱ��Ľṹ����������Ų����ɡ�����ṹ�����ʡ��縺�Եȣ��ѶȲ����������Ԫ�����ڱ��Ľṹ��ע��ͬ������ԭ�Ӿ�����۵���ߣ�

��ϰ��ϵ�д�
 Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�
�����Ŀ