��Ŀ����
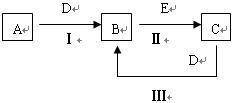
��11�֣���A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ��ʾ��
���� A����ʹʪ��ĺ�ɫ��ֽ���������壻C��D��Ϊ��������Ҫ�ɷ֣�E��һ����ɫ��ζ���ж����塣��д��E�Ļ�ѧʽ ��
A����ʹʪ��ĺ�ɫ��ֽ���������壻C��D��Ϊ��������Ҫ�ɷ֣�E��һ����ɫ��ζ���ж����塣��д��E�Ļ�ѧʽ ��
��д����Ӧ��Ļ�ѧ����ʽ ��
����A�ǵ���ɫ�����������D����ɫ���壻C�к��е����������Ӿ�Ϊ10 ��������
��������
��д��A�ĵ���ʽ
��д����Ӧ��Ļ�ѧ����ʽ
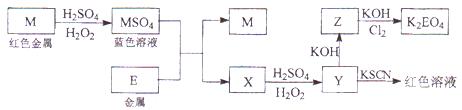
����A�ǵؿ��к������Ľ�����B�ڻ���������ʱ����ʻ�ɫ��B��C�о�����AԪ�أ���B��C����Һ�л��ʱ�����ɰ�ɫ����
��д��B�Ļ�ѧʽ ��д����Ӧ������ӷ���ʽ
����A�Ǻ�ɫ��ĩ������ ��B�ǻ���ɫ���壬C��Һ�ʼ��ԣ���Ư���ԡ�
��B�ǻ���ɫ���壬C��Һ�ʼ��ԣ���Ư���ԡ�
д����Ӧ������ӷ���ʽ ��

����
 A����ʹʪ��ĺ�ɫ��ֽ���������壻C��D��Ϊ��������Ҫ�ɷ֣�E��һ����ɫ��ζ���ж����塣��д��E�Ļ�ѧʽ ��
A����ʹʪ��ĺ�ɫ��ֽ���������壻C��D��Ϊ��������Ҫ�ɷ֣�E��һ����ɫ��ζ���ж����塣��д��E�Ļ�ѧʽ ����д����Ӧ��Ļ�ѧ����ʽ ��
����A�ǵ���ɫ�����������D����ɫ���壻C�к��е����������Ӿ�Ϊ10
 ��������
����������д��A�ĵ���ʽ
��д����Ӧ��Ļ�ѧ����ʽ
����A�ǵؿ��к������Ľ�����B�ڻ���������ʱ����ʻ�ɫ��B��C�о�����AԪ�أ���B��C����Һ�л��ʱ�����ɰ�ɫ����
��д��B�Ļ�ѧʽ ��д����Ӧ������ӷ���ʽ
����A�Ǻ�ɫ��ĩ������
 ��B�ǻ���ɫ���壬C��Һ�ʼ��ԣ���Ư���ԡ�
��B�ǻ���ɫ���壬C��Һ�ʼ��ԣ���Ư���ԡ�д����Ӧ������ӷ���ʽ ��
��11�֣����Ţ�CO ��1�֣� �� 4NH3+5O2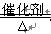 4NO+6H2O ��2�֣�
4NO+6H2O ��2�֣�
�Ƣ�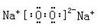 ��1�֣� ��Ca(OH)2+Na2CO3=CaCO3��+2NaOH ��2�֣�
��1�֣� ��Ca(OH)2+Na2CO3=CaCO3��+2NaOH ��2�֣�
�� ��NaAlO2��1�֣� ��Al3����4OH��=AlO2��+2H2O ��2�֣��� H��+Cl��+ClO��=Cl2��+H2O��2�֣�
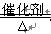 4NO+6H2O ��2�֣�
4NO+6H2O ��2�֣� �Ƣ�
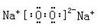 ��1�֣� ��Ca(OH)2+Na2CO3=CaCO3��+2NaOH ��2�֣�
��1�֣� ��Ca(OH)2+Na2CO3=CaCO3��+2NaOH ��2�֣��� ��NaAlO2��1�֣� ��Al3����4OH��=AlO2��+2H2O ��2�֣��� H��+Cl��+ClO��=Cl2��+H2O��2�֣�
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
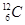 ����Ϊȷ��ԭ��������ԭ�ӣ�
����Ϊȷ��ԭ��������ԭ�ӣ�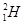 ��
��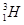 �������ⵯ�IJ��ϣ�
�������ⵯ�IJ��ϣ�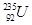 ������ԭ�ӵ��IJ��Ϻͺ˷�Ӧ�ѵ�ԭ�ϡ�ͬλ��ʾ�ٷ��㷺Ӧ���ڿ�ѧ�о�����ũҵ������ҽ�Ƽ������棬������
������ԭ�ӵ��IJ��Ϻͺ˷�Ӧ�ѵ�ԭ�ϡ�ͬλ��ʾ�ٷ��㷺Ӧ���ڿ�ѧ�о�����ũҵ������ҽ�Ƽ������棬������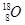 ��ǻ�����ȷ֤��������Ӧ�����̣�
��ǻ�����ȷ֤��������Ӧ�����̣�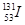 ���ڼ�״��������ܵ�ʵ��ȡ�����˵������ȷ����
���ڼ�״��������ܵ�ʵ��ȡ�����˵������ȷ����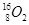 ��
��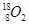 ��Ϊͬλ��
��Ϊͬλ��

 ��A��Ӧ��
��A��Ӧ��