��Ŀ����
( 9��)A��B���Ƕ�����Ԫ�أ�ԭ�����������Ų�ʽ�ֱ�Ϊ(n��1)sx��nsx+1npx+3��A��B���γɻ�����D��D����ˮʱ�������ݳ�����������ʹ�����ǵ�ľ����ȼ����ش��������⣺
(1)�Ƚϵ����ܣ�I1(A)________I1(B)(�>����<������ͬ)���Ƚϵ縺�ԣ�A________B��
(2)ͨ��AԪ�صĻ��ϼ���________����AԪ�س������ּ�̬���н��ͣ�
����ԭ�ӽṹ�Ĺ۵���н��ͣ� _______________________________________��
���õ����ܵĹ۵���н��ͣ� ___________________________________________��
��д��D��ˮ��Ӧ�����ӷ���ʽ��_______________________________________��
(1)�Ƚϵ����ܣ�I1(A)________I1(B)(�>����<������ͬ)���Ƚϵ縺�ԣ�A________B��
(2)ͨ��AԪ�صĻ��ϼ���________����AԪ�س������ּ�̬���н��ͣ�
����ԭ�ӽṹ�Ĺ۵���н��ͣ� _______________________________________��
���õ����ܵĹ۵���н��ͣ� ___________________________________________��
��д��D��ˮ��Ӧ�����ӷ���ʽ��_______________________________________��
( 9�� ) (1)��<����1�֣� ��<����1�֣� (2)��1�ۣ�1�֣�
������ԭ��ʧȥһ�����Ӻ��γ�1s22s22p6ʽ��ԭ�ӹ��ȫ�����ģ�1�������ӡ������ӽṹ��ϵ�����ͣ�������ʧȥ���ӣ�2�֣�
��Naԭ�ӵĵ�һ��������Խ�С���ڶ������ܱȵ�һ�����ܴ�ܶ��ͨ��Naԭ��ֻ��ʧȥһ������ ��2�֣���2Na2O2��2H2O===4Na����4OH����O2����2�֣�
������ԭ��ʧȥһ�����Ӻ��γ�1s22s22p6ʽ��ԭ�ӹ��ȫ�����ģ�1�������ӡ������ӽṹ��ϵ�����ͣ�������ʧȥ���ӣ�2�֣�
��Naԭ�ӵĵ�һ��������Խ�С���ڶ������ܱȵ�һ�����ܴ�ܶ��ͨ��Naԭ��ֻ��ʧȥһ������ ��2�֣���2Na2O2��2H2O===4Na����4OH����O2����2�֣�
������������ݹ���ԭ����֪��s����������2�����ӣ�����x��1��D����ˮʱ�������ݳ�����������ʹ�����ǵ�ľ����ȼ������D�ǹ������ƣ���n��2������A���ƣ�B����Ԫ�ء�
��1���ǽ�����Խǿ����һ������Խ�������Ƶĵ�һ������С����Ԫ�صġ�ͬ���縺��Ҳ���Ƶ�С����Ԫ�صġ�
��2����������������1����ͨ���ԣ�1�ۡ�
��������ԭ��ʧȥ1�����Ӻ��γ�1s22s22p6ʽ��ԭ�ӹ��ȫ�����ģ�1�������ӡ������ӽṹ��ϵ�����ͣ�������ʧȥ���ӣ�����ͨ���ԣ�1�ۡ�
������Naԭ�ӵĵ�һ��������Խ�С�����ڶ������ܱȵ�һ�����ܴ�ܶ��ͨ��Naԭ��ֻ��ʧȥһ�����ӣ�����ԣ�1�ۡ�
�۹�����������ˮ�����������������ƣ���Ӧ�����ӷ���ʽ��2Na2O2��2H2O===4Na����4OH����O2����
���������ж�ԭ�Ӻ�����ӵ��Ų�ʱ��Ӧ�����ù���ԭ�����з��������Ȿ��Ĺؼ���������ʹ�����ǵ�ľ����ȼ���������������̶���һ�������ɡ�

��ϰ��ϵ�д�
 Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
 ��
�� 
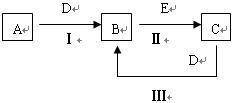
 A����ʹʪ��ĺ�ɫ��ֽ���������壻C��D��Ϊ��������Ҫ�ɷ֣�E��һ����ɫ��ζ���ж����塣��д��E�Ļ�ѧʽ ��
A����ʹʪ��ĺ�ɫ��ֽ���������壻C��D��Ϊ��������Ҫ�ɷ֣�E��һ����ɫ��ζ���ж����塣��д��E�Ļ�ѧʽ �� ��������
�������� ��B�ǻ���ɫ���壬C��Һ�ʼ��ԣ���Ư���ԡ�
��B�ǻ���ɫ���壬C��Һ�ʼ��ԣ���Ư���ԡ�

 �Խ�С�ڷ���
�Խ�С�ڷ��� �� �ֲ�ͬ�ܼ��ĵ��ӡ�
�� �ֲ�ͬ�ܼ��ĵ��ӡ�