��Ŀ����
11��ij��ѧѧϰС��ͬѧ�����һ��ʵ��װ������ⱥ��ʳ��ˮ���������������������������Ԥ��H2�����6 0mL���ң�ͬʱ���������������ԣ��������µ������ɹ�ѡ����1��Pt����ֱ����Դ��������
��2��ѡ���Ҫ��������һ��װ�ã��ﵽ����ʵ��Ŀ�ģ���д���������ӿڵ�����˳�����ţ���A���ν�GFH��B���ν�DEC��
��3����˵���������������Ե�ʵ�������ǵ��۵⻯����Һ����ɫ��
��4������װ��50mL����ʳ��ˮ���ǰ����Һ����仯�ɺ��ԣ���������ȫ���ݳ�������õ�����Ϊ5.6mL����״����ʱֹͣͨ�磮ҡ��U�ι��ڵ���Һ����Һ��pHԼΪ12.5��

���� ��1������ʵ��Ŀ����������������������ͭӦΪ������PtΪ������
��2������ʵ���Ŀ�ĺ�װ�õ�����������װ�ã�
��3����������ʹ���۵⻯����Һ����ɫ˵����
��4�����ݵ�ⱥ��ʳ��ˮ�ķ���ʽ��2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2�������ù�ʽC=$\frac{n}{V}$������NaOH�����ʵ���Ũ�ȣ�Ȼ����������ӵ�Ũ�ȣ�������pH��
��� �⣺��1������ʵ��Ŀ����������������������ͭӦΪ������PtΪ����������Pt��ֱ����Դ���������ʴ�Ϊ������
��2���������������������ˮ��������Ԥ��H2�����60ml���ң�����ѡH��ѡI�������Ƕ̽�����������A��G����װ�е��۵⻯����Һ��ϴ��ƿ��������ʱ������Ҫ�����̳�������B��D������Ҫ����β����������E��C���ʴ�Ϊ��GFH��DEC��
��3����������ʹ���۵⻯����Һ����ɫ˵���������������ԣ��ʴ�Ϊ�����۵⻯����Һ����ɫ��
��4�����ⱥ��ʳ��ˮ�ķ���ʽ��2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+H2��+Cl2������������H2�����Ϊ16.8mL��0.00075molʱ�������������Ƶ����ʵ���Ϊ0.0015mol��������Һ��NaOH�����ʵ���Ũ��=$\frac{0.0015mol}{0.05L}$�T0.03mol/L�����������ӵ�Ũ��Ϊ$\frac{1{0}^{-14}}{0.03}$=$\frac{1}{3}$��10-12mol/L��pH=12.5���ʴ�Ϊ��12.5��
���� ������Ҫ�����˵�ⱥ��ʳ��ˮ�IJ�����Ҫ����ʵ���ԭ���Ͳ���������������ѧ����������������

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�| A�� | ������ΪNA��CO��N2������������Ϊ28g | |
| B�� | 0.1mol������Ϊ�������õ����ӵ���Ŀһ��Ϊ0.4NA | |
| C�� | 22.4L�����͵����Ļ�����庬��2NA��ԭ�� | |
| D�� | 0.1mol•L-1������������Һ����0.1NA��OH- |
| A�� | �ڱ�״���£�1mol �κ����ʵ����ԼΪ22.4L | |
| B�� | 22.4L���������ʵ���һ����1mol | |
| C�� | �ڱ�״����0.5mol �����������Ļ������������ԼΪ11.2L | |
| D�� | ���1mol��������Ϊ22.4L������Щ����һ�����ڱ�״�� |
| A�� | �������������е������Ӻ������ӿ�����������ȥ | |
| B�� | �Ȼ������������Ȼ�泥��ӹ������ռ���Һ��������� | |
| C�� | ���������л����������ᣬ�ñ���̼������Һϴ�Ӻ��Һ | |
| D�� | Ӳ֬��������л��������ĸ��ͣ�����������ȥ |
| A�� | 12 | B�� | 11 | C�� | 7 | D�� | 3 |
| A�� | XO3-��XΪ+5�ۣ���XԪ��λ�ڵ�VA�� | |
| B�� | Xλ�ڵڶ����ڵ�VIIA�� | |
| C�� | X��������Ԫ�� | |
| D�� | X�����ǵ�Ԫ�� |
| A�� | ��ͬ��ͬѹ�£���ͬ������κ�����������ԭ������ͬ | |
| B�� | ��ͬ�����£�N2��O2�Ļ��������������N2����ԭ������� | |
| C�� | �����ʵ�����NH4+��OH-��������������ҵ�����Ҳ��� | |
| D�� | 1mol �һ��к��еĵ�����Ϊ18NA |
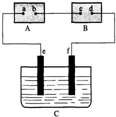 ͼ��AΪ��Դ��BΪ������ʳ��ˮ�ͷ�̪��Һ����ֽ��CΪʢ��ϡ����ĵ��ۣ�e��fΪ���缫����ͨ��·����B�ϵ�c���Ժ�ɫ���Իش�
ͼ��AΪ��Դ��BΪ������ʳ��ˮ�ͷ�̪��Һ����ֽ��CΪʢ��ϡ����ĵ��ۣ�e��fΪ���缫����ͨ��·����B�ϵ�c���Ժ�ɫ���Իش�