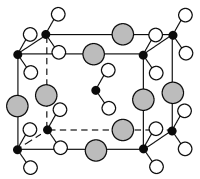
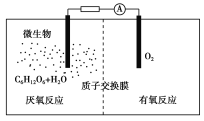
��Ŀ����
����Ŀ��T��ʱ���мס��������ܱ������������������Ϊ1L�������������Ϊ2L���ֱ���ס����������м���6molA��3molB��������Ӧ���£�3A��g����bB��g��![]() 3C��g����2D��g����H<0;4minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬
3C��g����2D��g����H<0;4minʱ�������ڵķ�Ӧǡ�ôﵽƽ�⣬
A��Ũ��Ϊ2.4mol/L��B��Ũ��Ϊ1.8mol/L;tminʱ�������ڵķ�Ӧ��ƽ�⣬
B��Ũ��Ϊ0.8mol/L.���������Ϣ�ش��������⣺
��1���������з�Ӧ��ƽ������v��B����______________��
��2���������з�Ӧ�ﵽƽ��ʱ����ʱ��t__________4 min������ڡ�����С�ڡ����ڡ�����
��3����Ҫʹ�ס���������B��ƽ��Ũ����ȣ����Բ�ȡ�Ĵ�ʩ��______________��
A�������¶Ȳ��䣬����������������2 L
B����������������䣬ʹ�����������¶�
C����������������¶ȶ����䣬����м���һ������A����
D����������������¶ȶ����䣬����м���һ������B����
��4�����¶��£������Ϊ1L���ܱ�������ͨ��A��B��C��D�������ʵ����ֱ�Ϊ3mol��1mol��3mol��2mol����ʱ��Ӧ________________������ڻ�ѧƽ��״̬������������Ӧ������С������淴Ӧ������С�����
���𰸡���1��0.3mol/��L��min����2��������3��AC��4��������Ӧ�������
��������
���������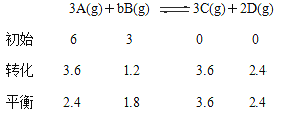
��1���������з�Ӧ��ƽ������v��B��=![]() 0.3 mol/��L��min����
0.3 mol/��L��min����
��2����������������ڼף���������ѹǿС�ڼף��ҷ�Ӧ����С�ڼף���Ӧ�ﵽƽ��ʱ����ʱ��t ����4 min����3������A��B�ı仯��Ϊ3.6��1.2����֪b=1����������ȣ��൱�ڼ�ѹ��ƽ�������ƶ���B��ƽ��Ũ���״����ҡ�A�������¶Ȳ��䣬����������������2 L���ס���Ϊ��Чƽ�⣻B����������������䣬ʹ�����������¶���ƽ�������ƶ�������B��ƽ��Ũ������C����������������¶ȶ����䣬����м���һ������A������ƽ�������ƶ���B��ƽ��Ũ����С���п��ܵ����ң�D����������������¶ȶ����䣬����м���һ������B������B��ƽ��Ũ���������ܵ����ҡ���4�� ���¶���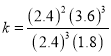 �������Ϊ1L���ܱ�������ͨ��A��B��C��D�������ʵ����ֱ�Ϊ3mol��1mol��3mol��2mol��
�������Ϊ1L���ܱ�������ͨ��A��B��C��D�������ʵ����ֱ�Ϊ3mol��1mol��3mol��2mol��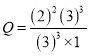 ��
��![]() ������ʱ��Ӧ������Ӧ���������
������ʱ��Ӧ������Ӧ���������

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�