جâؤ؟ؤعبف
،¾جâؤ؟،؟دضسذ1Lخ´ضھ³ة·ضµؤ»ى؛دبـز؛£¬ئنضذ³ءث؛¬سذ0.2mol/LµؤNa+ح⣬»¹؟ةؤـ؛¬سذدآءذہë×سضذµؤز»ضض»ٍ¼¸ضض£؛
![]()
دض½ّذذبçدآتµرé²ظ×÷£¨أ؟´ختµرéثù¼ستش¼ء¾ù¹ء؟£©£؛
زرضھ:¢ظ![]() £¬¢ع
£¬¢ع![]() £¬Cl2شع³£خآدآخھ»ئآجة«ئّجه£¬؟ةبـسعث®£¬ئنث®بـز؛خھا³»ئآجة«£»Br2شع³£خآدآتاةî؛ى×طة«ز؛جه£¬خ¢بـسعث®£¬ئنث®بـز؛خھ»ئة«،£
£¬Cl2شع³£خآدآخھ»ئآجة«ئّجه£¬؟ةبـسعث®£¬ئنث®بـز؛خھا³»ئآجة«£»Br2شع³£خآدآتاةî؛ى×طة«ز؛جه£¬خ¢بـسعث®£¬ئنث®بـز؛خھ»ئة«،£
£¨1£©سةئّجهB؟ةب·¶¨´²âز؛ضذ؛¬سذµؤہë×ستا____________،£
£¨2£©سة³ءµيD؛ح³ءµيE؟ةزشإذ¶د´²âز؛ضذز»¶¨؛¬سذ_____ہë×س£¬¾ف´ث؟ةزشإإ³µؤہë×ستا_____،£
£¨3£©سة°×ة«³ءµيB؟ةب·¶¨´²âز؛ضذ؛¬سذµؤہë×ستا____£¬ذ´³ِ²ْةْ³ءµيBµؤہë×س·½³جت½_______،£
£¨4£©ؤ³ح¬ر§¶ءح¼؛َ£¬بدخھ´²âز؛ضذز»¶¨²»؛¬Br،ھ£¬ثûµؤإذ¶دزہ¾فتا______،£
£¨5£©×غ؛د·ضخِ£¬´²âز؛ضذ![]() µؤخïضتµؤء؟إ¨¶بµؤب،ضµ·¶خ§خھ________،£
µؤخïضتµؤء؟إ¨¶بµؤب،ضµ·¶خ§خھ________،£
،¾´ً°¸،؟NH4+ SO42-،¢CO32- Mg2+،¢Ba2+،¢Fe3+ HCO3- HCO3-+OH-+Ba2+=BaCO3،+H2O Br- ´َسعµبسع0.1mol/L
،¾½âخِ،؟
´²âز؛؛حآب»¯±µبـز؛·´س¦µأµ½³ءµيA£¬شٍبـز؛ضذ؟ةؤـ؛¬سذCO32-،¢SO32-،¢SO42-£¬دٍ³ءµيضذ¼سبëد،دُثلةْ³ةئّجه£¬ازسذ²؟·ض³ءµي²»بـ½â£¬شٍبـز؛ضذ´وشعCO32-£¬؟ةؤـ´وشعSO32-،¢SO42-ضذµؤء½ضض»ٍز»ضض£¬¸ù¾فہë×س¹²´وضھ£¬بـز؛ضذ²»´وشعBa2+£»آثز؛AضذسذBa2+£¬¼سبë¹ء؟µؤNaOHبـز؛µأµ½ئّجهB،¢°×ة«³ءµيB£¬شٍبـز؛ضذز»¶¨؛¬سذNH4+،¢HCO3-£¬ز»¶¨²»´وشعFe3+£¬ئّجهBخھNH3£¬°×ة«³ءµيBخھBaCO3£¬آثز؛Bضذ¼سبëدُثلزّ،¢دُثلبـز؛µأµ½°×ة«³ءµيC£¬CخھAgCl£¬ثµأ÷آثز؛Bضذ؛¬سذCl-£¬سةسع¼سبëآب»¯±µبـز؛£¬²»ؤـب·¶¨شبـز؛ضذتا·ٌ؛¬سذCl-£¬¾ف´ث·ضخِ،£
£¨1£©¼سبëNaOHبـز؛؛َؤـ²ْةْئّجه£¬؟ةضھ¸أئّجهخھNH3£¬¹ت´²âز؛ضذ؛¬سذNH4+£¬¹ت´ً°¸خھ£؛NH4+£»
£¨2£©¼سبëBaCl2ةْ³ة²؟·ض²»بـسعد،دُثلµؤ°×ة«³ءµي£¬شظ×غ؛د±ي¸ٌثù¸ّµؤزُہë×س؟ةضھ´²âز؛ضذ؛¬سذSO42-£¬سضزٍخھ²؟·ض°×ة«³ءµيؤـبـسعدُثل²¢²ْةْخقة«ئّجه£¬؟ةضھ´²âز؛ضذ»¹؛¬سذCO32-£¬¹ت´²âز؛ضذز»¶¨؛¬سذSO42-،¢CO32-£»زٍخھرôہë×سضذMg2+،¢Ba2+،¢Fe3+،¢»لسëCO32-·´س¦ةْ³ة³ءµي£¬¹ت´²âز؛ضذز»¶¨²»؛¬Mg2+،¢Ba2+،¢Fe3+£¬¹ت´ً°¸خھ£؛SO42-،¢CO32-£»Mg2+،¢Ba2+،¢Fe3+£»
£¨3£©¼سبëBaCl2بـز؛²»²ْةْ³ءµي£¬آثز؛¼سبëNaOHبـز؛؛َسضةْ³ة°×ة«³ءµي£¬؟ةحئ³ِبـز؛ضذ²»؛¬Mg2+£¬¹ت؟ةضھ°×ة«³ءµيخھBaCO3£¬¹ت´²âز؛ضذ؛¬سذHCO3-£»²ْةْ³ءµيBµؤہë×س·½³جت½خھ£؛HCO3-+OH-+Ba2+=BaCO3،+H2O£¬¹ت´ً°¸خھ£؛HCO3-£»HCO3-+OH-+Ba2+=BaCO3،+H2O£»
£¨4£©نهث®خھ³بة«ز؛جه£¬نه»¯زّخھµ»ئة«¹ججه£¬¶ّبـز؛Bضذح¨بëآبئّ£¬بـز؛³تا³آجة«ازبـز؛Bµخ¼سدُثلثل»¯µؤدُثلزّبـز؛£¬³ِدض°×ة«³ءµي£¬¹ت´²âز؛ضذ²»؛¬Br-£¬¹ت´ً°¸خھ£؛Br-£»
£¨5£©°×ة«³ءµيBخھBaCO3£¬ئّجهBخھNH3£¬°×ة«³ءµيEخھCaCO3£¬°×ة«³ءµيDخھBaSO4£¬¹ت؟ةضھ´²âبـز؛ضذ؛¬سذ0.1molHCO3-،¢0.1molNH4+،¢0.1molCO32-،¢0.05molSO42-£¬´²âز؛ضذتا·ٌ؛¬سذCl-²»ؤـب·¶¨£¬¼ظةè²»؛¬Cl-£¬شٍ¸ù¾فبـز؛µçضذذشششٍ؟ةµأ´²âز؛ضذK+×îذ،إ¨¶بخھ0.1mol/L£¬¹ت´ً°¸خھ£؛´َسعµبسع0.1mol/L،£

 ذ،ر§¶ل¹عAB¾يدµءذ´ً°¸
ذ،ر§¶ل¹عAB¾يدµءذ´ً°¸،¾جâؤ؟،؟700،وت±£¬دٍبف»خھ2Lµؤأـ±صبفئ÷ضذ³نبëز»¶¨ء؟µؤCO؛حH2O£¬·¢ةْ·´س¦£؛CO(g)£«H2O(g) ![]() CO2(g)£«H2(g)£¬·´س¦¹³جضذ²â¶¨µؤ²؟·ضت¾ف¼ûدآ±ي(±يضذt2>t1)
CO2(g)£«H2(g)£¬·´س¦¹³جضذ²â¶¨µؤ²؟·ضت¾ف¼ûدآ±ي(±يضذt2>t1)
·´س¦ت±¼ن/min | n(CO)/mol | n(H2O)/mol |
0 | 1.20 | 0.60 |
t1 | 0.80 | |
t2 | 0.20 |
دآءذثµ·¨صب·µؤتا(،،،،)
A.·´س¦شعt1minؤعµؤئ½¾ùثظآتخھv(H2)£½![]() mol،¤L£1،¤min£1
mol،¤L£1،¤min£1
B.±£³ضئنثûجُ¼²»±ن£¬ئًت¼ت±دٍبفئ÷ضذ³نبë0.60mol CO؛ح1.20mol H2O£¬´ïµ½ئ½؛âت±n(CO2)£½0.40mol
C.±£³ضئنثûجُ¼²»±ن£¬دٍئ½؛âجهدµضذشظح¨بë0.20mol H2O£¬سëشئ½؛âدà±ب£¬´ïµ½ذآئ½؛âت±CO×ھ»¯آتشِ´َ£¬H2Oµؤجه»·ضت¼ُذ،
D.بôخآ¶بة¸كضء800،و£¬ةدتِ·´س¦ئ½؛â³£تخھ0.64£¬شٍص·´س¦خھخüبب·´س¦
،¾جâؤ؟،؟2019ؤê4شآ23بص£¬ضذ¹ْبثأٌ¾ü³ةء¢70ضـؤê،£جلµ½؛£¾ü¾ح²»µأ²»جل؛½؟صؤ¸½¢£¬خز¹ْصشع½¨شىµعبثز؛½؟صؤ¸½¢،£؛½ؤ¸µؤءْ¹ازھؤح³ه»÷£¬¼×°هزھؤح¸كخآ£¬حâ؟ازھؤح¸¯ت´£®
(1)ؤّ¸ُ¸ض؟¹¸¯ت´ذشؤـا؟£¬»ùج¬C r ش×س¼غ²مµç×سµؤµç×سإإ²¼ت½خھ_______________،£
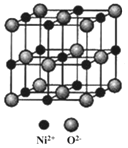
(2)؛½ؤ¸¼×°هح؟سذز»²مؤح¸كخآµؤ²ؤءد¾غ¹èرُحé(½ل¹¹بçح¼ةêثùت¾)،£»ùج¬Siش×سµç×سص¼¾ف×î¸كؤـ¼¶µؤµç×سشئآضہھح¼خھ_______ذخ£؛H،¢C،¢O،¢Si ثؤضضشھثطضذµؤµç¸؛ذش×î¸كµؤتا______،£
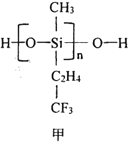
(3)؛£رَتاشھثطµؤز،ہ؛£¬؛£ث®ضذ؛¬سذ´َء؟آ±×هشھثط،£
¢ظîرآ±»¯خïµؤبغµمبçدآ±يثùت¾£؛
TiF4 | TiCl4 | TiBr4 | TiI4 | |
بغµم/،و | 377 | -24 | 38 | 150 |
½âتح±يضذآ±»¯خïض®¼نبغµم²îزىµؤشزٍتا________________________،£
¢عOF2µؤ؟ص¼ن¹¹ذحخھ___________£¬ئنضذOش×سشس»¯·½ت½خھ__________شس»¯،£
¢غآبشھثط؟ةزشذخ³ة¶àضض؛¬رُثل£¬ئنثلذشسةبُµ½ا؟µؤث³ذٍخھ£؛HClO <HClO2<HClO3<HClO4،£تش½âتحئنثلذشا؟بُµؤشزٍتا_______________ ،£
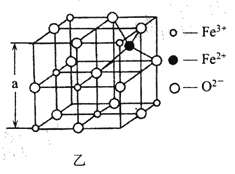
(4)؛£µ×½ًتôبيؤàتاشع؛£رَµ×¸²¸ا×إµؤز»²م؛ى×طة«³ء»خشج²ط´َ×إ¼«µؤ×تش´£¬؛¬سذ¹è،¢رُ،¢أج،¢ذ؟µب،£بçح¼تا´سجْرُجهہë×س¾§جهFe3O4 ضذب،³ِµؤؤـجهدضئن¾§جه½ل¹¹µؤز»¸ِء¢·½جه،£
¢ظ¾§جهضذµؤرُہë×ستا·ٌ¹¹³ةءثأوذؤء¢·½×îأـ¶ر»£؟________(جî،°تا،±»ٍ،°·ٌ،±)£»
¢عزرضھ£؛Fe3O4 ¾§جهµؤأـ¶بخھ5. 18gcm -3, ¸ù¾ف¸أح¼¼ئثمa________nrn (ذ´³ِ¼ئثمت½¼´؟ة£¬°¢·ü¼سµآآق³£تµؤضµخھN A)