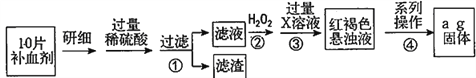
��Ŀ����
����Ŀ��ij���������Ҫ�ɷ�ΪAl2O3��xH2O��������Fe2O3��SiO2���ʡ���ȡ17.5g��������Ʒ������200mL1.65mol/LϡH2SO4��ǡ����ȫ��Ӧ�����˵õ�����0.3g��Ȼ������Һ�м�������NaOH��Һ���õ�����2.14g������ʾ��SiO2���ʲ���ϡ���ᷴӦ��
��1�������Ʒ��Fe2O3�����ʵ�����____��
��2���Լ�����Ʒ��Al2O3��������____��
��3���Լ�����Ʒ��Al2O3��xH2O��xֵ��____��
���𰸡�0.01mol 10.2g 3
��������
�������������������ᷴӦ�����������費��Ӧ����0.3gΪ�����������������Һ�м����������������ƣ�������ת��Ϊƫ�����Σ��ʵõ�����2.14gΪ����������������
��1������FeԪ���غ㣬n��Fe2O3��=![]() nFe(OH)3=
nFe(OH)3=![]() ��
��![]() =0.01mol��
=0.01mol��
����0.01mol��
��2������������غ㣺3n[Al2(SO4)3]+3n[Fe2(SO4)3]=n(H2SO4)����3n[Al2(SO4)3]+3��0.01mol
=0.2L��1.65mol/L����n[Al2(SO4)3]=0.1mol������AlԪ���غ㣬n��Al2O3��=n[Al2��SO4��3]=0.1mol����m��Al2O3��=0.1mol��102g/mol=10.2g��
����10.2g��
��4����Ʒ��ˮ������Ϊ17.5g-0.3g-10.2g-0.01mol��160g/mol=5.4g�����ʵ���Ϊ![]() =0.3mol����0.1mol��0.3mol=1��x����x=3��
=0.3mol����0.1mol��0.3mol=1��x����x=3��
����3��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���������ǽ��������У���������Ҫ��ɫ�����������֪ʶ�ش��������⣺
(1)���зdz���Ҫ����;����д�����е�һ�֣�_________________________________��
(2)�Ŵ����������ϳɷ�һֱ�Ǹ��գ���������ѧ�Ҳŵ�֪���Ϊ�����Σ�������ɫ�Ĺ���ͭ��(BaCuSi2Ox��ͭΪ��2��)�����й��ڹ���ͭ����˵������ȷ����________��
A��������������ʽ��ʾΪBaO��CuO��2SiO2
B�������ȶ���������ɫ
C��x����6
D�����ܽ���ǿ���ǿ��
(3)��ҵ���ᴿ���ж���·�ߣ�����һ�ֹ�������ʾ��ͼ���£�
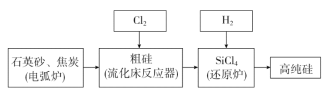
���ڵ绡¯�з����ķ�Ӧ��Ҫ�ڸ����������У�д���÷�Ӧ��ѧ����ʽ��________________��
��SiCl4����ˮ�⣬�ڿ����������̳��Ͱ������Ʋ���д��ˮ��Ļ�ѧ����ʽΪ____________________��
������������Ӧ�IJ����У���SiCl4�⣬����SiHCl3��SiH2Cl2��SiH3Cl��FeCl3�ȣ��й����ʵķе��������±�������SiCl4���������ʵķ���Ϊ________��
���� | Si | SiCl4 | SiHCl3 | SiH2Cl2 | SiH3Cl | HCl | SiH4 |
�е�/�� | 2355 | 57.6 | 31.8 | 8.2 | ��30.4 | ��84.9 | ��111.9 |