��Ŀ����
����Ŀ�����������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ����(K2Cr2O7)������ͼ��

��֪����Na2CO3��Al2O3![]() 2NaAlO2��CO2����
2NaAlO2��CO2����
��Cr2O72-��H2O![]() 2CrO42-��2H����
2CrO42-��2H����
��������ش��������⣺
(1)�����ա�ʱFeO��Cr2O3ת��ΪNa2CrO4��Fe2O3��д�������ա�ʱ��Ӧ�Ļ�ѧ����ʽ��_____________��
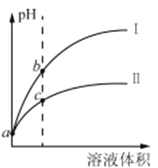
(2)��Ҫ��⡰�ữ����������Һ��pH�Ƿ����4.5��Ӧ��ʹ��______(����ĸ����)��
a���㷺pH��ֽ��
b������
c��pH��
d������pH��ֽ
�ڡ��ữ�������ô��������ҺpH��5����Ŀ����_____________��
(3)���������ɶಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢_____________��_____________�����ˡ�ϴ�ӡ����
(4)������ʵ��ܽ���������±���ʾ�����������з�����Ӧ�Ļ�ѧ����ʽΪNa2Cr2O7��2KCl===K2Cr2O7����2NaCl���÷�Ӧ����Һ���ܷ�����������_____________��
���� | KCl | NaCl | K2Cr2O7 | Na2Cr2O7 |
�ܽ�� | ||||
(g/100 gˮ) | ||||
0 �� | 28 | 35.7 | 4.7 | 163 |
40 �� | 40.1 | 36.4 | 26.3 | 215 |
80 �� | 51.3 | 38 | 73 | 376 |
(5)��ȡ�ظ��������5.000 g���500 mL��Һ��ȡ��50.00 mL�ڵ���ƿ�У�����20 mL 2 mol/L H2SO4��Һ�������⻯�أ����ڰ���5 min��Ȼ�����100 mLˮ������3mL__________(���Լ�����)��ָʾ������0.100 0 mol/L Na2S2O3����Һ���еζ�����ʵ���й���ȥNa2S2O3����Һ48.00 mL�������ò�Ʒ���ظ���صĴ���Ϊ___________(����3λ��Ч����)��(��֪��Cr2O72-��6I����14H��===2Cr3����3I2��7H2O��I2��2S2O32-===2I����S4O62-)
���𰸡�4FeO��Cr2O3��8Na2CO3��7O2![]() 8Na2CrO4��2Fe2O3��8CO2 cd ʹCrO42-ת��ΪCr2O72- ����Ũ�� ��ȴ�ᾧ ��ͬ�¶���K2Cr2O7���ܽ�ȱ�Na2Cr2O7��С(��ϵ��¶��£�����������K2Cr2O7���ܽ����С) ������Һ 47.0%
8Na2CrO4��2Fe2O3��8CO2 cd ʹCrO42-ת��ΪCr2O72- ����Ũ�� ��ȴ�ᾧ ��ͬ�¶���K2Cr2O7���ܽ�ȱ�Na2Cr2O7��С(��ϵ��¶��£�����������K2Cr2O7���ܽ����С) ������Һ 47.0%
��������
��������̼���ƻ��ͨ�����գ��õ�Na2CrO4��Fe2O3��MgO��NaAlO2�Ļ����ϵ��Ȼ���ˮ�ܽ�õ�����Fe2O3��MgO��Na2CrO4��NaAlO2��Һ���ٵ�����Һ��pH��ʹƫ��������ȫ����������������Һ��pHʹCrO42-ת��ΪCr2O72-�������������Һ�м����Ȼ��أ������ܽ�Ƚ�С��K2Cr2O7��
��1�����ݻ��ϼ���������Ԫ���غ����ƽ��ѧ����ʽ��
��2����ͨ��pH��ֻֽ�ܲⶨ��ҺpH���������ⶨpH��С������Ҫ�þ�ȷ��ֽ��pH�ƣ�
�ڽ������ͼ�ͷ�Ӧ�����еõ����ʷ������ữ�����ô��������ҺpH��5Ϊ��ת��CrO42-����ΪCr2O72-��
��3����Һ�еõ����ʾ���ķ���������Ũ������ȴ�ᾧ������ϴ�Ӳ�������ش�
��4�����������ܽ�ȱȽϷ�������Ӧ���ܽ��С�ķ�����У�
��5���ⵥ���������۱�������Ӧ�Ĺ�ϵʽΪCr2O72-��3I2��62S2O32-���ɴ˼��㼴����
��1�����ݷ�Ӧ�и����ʵ�Ԫ�ػ��ϼ۱仯��֪�����ӣ�2�۱�Ϊ��3�ۣ����ӣ�3�۱�Ϊ��6�ۣ�����0�۱�Ϊ��2�ۣ����ݻ��ϼ���������Ԫ���غ����ƽ��ѧ����ʽΪ
4FeOCr2O3+8Na2CO3+7O2![]() 8Na2CrO4+2Fe2O3+8CO2�����ʴ�Ϊ��4FeOCr2O3��8Na2CO3��7O2
8Na2CrO4+2Fe2O3+8CO2�����ʴ�Ϊ��4FeOCr2O3��8Na2CO3��7O2![]() 8Na2CrO4��2Fe2O3��8CO2����
8Na2CrO4��2Fe2O3��8CO2����
��2����Ҫ����ữ��������Һ��pH�Ƿ����4.5����ͨpH��ֻֽ�ܲⶨ��ҺpH���������ǽ��Ʋⶨ��ȷ�ⶨ��Ҫ��pH�ƻ�ȷpH��ֽ���ʴ�Ϊ��cd��
���ữ�����ô��������ҺpH��5����������ͼ�����ʵ�ת�����Ʊ�Ŀ�Ŀ�֪����Ϸ�Ӧƽ��Cr2O72-+H2O2CrO42-+2H+�������ᣬ������Ũ������ƽ�����ƣ�������ʹCrO42-ת��ΪCr2O72-���ʴ�Ϊ��ʹCrO42-ת��ΪCr2O72-��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ����壬�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��4������ͼ�����ʵ��ܽ�ȷ����Աȣ�����������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7+2KCl=K2Cr2O7��+2NaCl��˵����ͬ�¶���K2Cr2O7���ܽ�ȱ�Na2Cr2O7��С(��ϵ��¶��£�����������K2Cr2O7���ܽ����С)��
�ʴ�Ϊ����ͬ�¶���K2Cr2O7���ܽ�ȱ�Na2Cr2O7��С(��ϵ��¶��£�����������K2Cr2O7���ܽ����С)��
��5��������������ɫ����˿����õ�����ָʾ��������Cr2O72-��6I����14H��=2Cr3����3I2��7H2O��I2��2S2O32-=2I����S4O62-�ɵ÷�Ӧ�Ĺ�ϵʽΪ��Cr2O72-��3I2��62S2O32-������n(Cr2O72-)=0.1000mol/L��0.048L/6=0.0008mol������500mL�ظ������n(Cr2O![]() )=0.008mol��m(K2Cr2O7)=0.008mol��294gmol��1=2.352g���䴿��=2.352g/5g��100%=47%���ʴ�Ϊ��������Һ��47.0%��
)=0.008mol��m(K2Cr2O7)=0.008mol��294gmol��1=2.352g���䴿��=2.352g/5g��100%=47%���ʴ�Ϊ��������Һ��47.0%��

 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�����Ŀ���±���Ԫ�����ڱ������ڵ�һ����
�� | ||||||||
�� | �� | �� | ||||||
�� | �� | �� | �� | |||||
(1)��-��Ԫ���н�������ǿ��Ԫ��λ�����ڱ��е�λ��Ϊ______________________��
(2)������γɵĻ���������ˮ����ҷ�Ӧ���ɰ�ɫ����������A,��д���÷�Ӧ�Ļ�ѧ����ʽ ___________________ ������A��ʵ�����Ʊ���ѧ����ʽΪ______________��
(3)�����γɵĵ�����ˮ��Ӧ�ķ���ʽ _____________���÷�Ӧ��������_______��Ӧ��
(4)�ڡ��ۡ��ߵ���ۺ����������������ǿ��˳����__________________���û�ѧʽ��ʾ������������ĸ�Ԫ���������������γɵ���̬�⻯���ȶ�����ǿ������˳���ǣ��û�ѧʽ��ʾ��__________________��
(5)����Ԫ�آۡ�����������������ˮ�������Ӧ�����ӷ���ʽΪ_____________��