��Ŀ����
�ҹ��Ǹ��������,���������������һλ,��¯��������Ϊ�ձ������������ij�ֿ�ʯ����Ԫ����������FemOn����ʽ����,�ֽ�������ʵ��:����������ʯ��Ʒ����,��ȡ25.0 g��Ʒ���ձ���,����ϡ�������ܽ�,�����ϼ��ȡ�����,��ȥ�������������Һ�м���10.0 gͭ�۳�ַ�Ӧ����ˡ�ϴ�ӡ������ʣ�����3.6 g��ʣ����Һ��2 mol��L-1������KMnO4�ζ�,���յ�ʱ����KMnO4��Һ���25.0 mL��
(1)���������ʯ����Ԫ�ص�����������
(2)����������FemOn�Ļ�ѧʽ(m��nΪ������)��
(1)���������ʯ����Ԫ�ص�����������
(2)����������FemOn�Ļ�ѧʽ(m��nΪ������)��
(1)56%��(2)Fe5O7
(1)����8H++Mn +5Fe2+
+5Fe2+ Mn2++5Fe3++4H2O��֪n(Fe2+)=5n(Mn
Mn2++5Fe3++4H2O��֪n(Fe2+)=5n(Mn )="5��0." 025 L��2 mol��L-1="0.25" mol,m(Fe)="14" g,����ʯ����Ԫ�ص���������=
)="5��0." 025 L��2 mol��L-1="0.25" mol,m(Fe)="14" g,����ʯ����Ԫ�ص���������= ��100%=56%;(2)�μӷ�Ӧn(Cu)=
��100%=56%;(2)�μӷ�Ӧn(Cu)= ="0.1" mol,����2Fe3++Cu
="0.1" mol,����2Fe3++Cu 2Fe2++Cu2+��֪����ʯ��n(Fe3+)="0.2" mol��n(Fe2+)="0.25" mol-0.2 mol="0.05" mol,1������������Ӻ���һ��+2����ԭ��,����4��+3����ԭ��,���ݻ��ϼۿ�֪Oԭ��Ϊ7��
2Fe2++Cu2+��֪����ʯ��n(Fe3+)="0.2" mol��n(Fe2+)="0.25" mol-0.2 mol="0.05" mol,1������������Ӻ���һ��+2����ԭ��,����4��+3����ԭ��,���ݻ��ϼۿ�֪Oԭ��Ϊ7��
 +5Fe2+
+5Fe2+ Mn2++5Fe3++4H2O��֪n(Fe2+)=5n(Mn
Mn2++5Fe3++4H2O��֪n(Fe2+)=5n(Mn )="5��0." 025 L��2 mol��L-1="0.25" mol,m(Fe)="14" g,����ʯ����Ԫ�ص���������=
)="5��0." 025 L��2 mol��L-1="0.25" mol,m(Fe)="14" g,����ʯ����Ԫ�ص���������= ��100%=56%;(2)�μӷ�Ӧn(Cu)=
��100%=56%;(2)�μӷ�Ӧn(Cu)= ="0.1" mol,����2Fe3++Cu
="0.1" mol,����2Fe3++Cu 2Fe2++Cu2+��֪����ʯ��n(Fe3+)="0.2" mol��n(Fe2+)="0.25" mol-0.2 mol="0.05" mol,1������������Ӻ���һ��+2����ԭ��,����4��+3����ԭ��,���ݻ��ϼۿ�֪Oԭ��Ϊ7��
2Fe2++Cu2+��֪����ʯ��n(Fe3+)="0.2" mol��n(Fe2+)="0.25" mol-0.2 mol="0.05" mol,1������������Ӻ���һ��+2����ԭ��,����4��+3����ԭ��,���ݻ��ϼۿ�֪Oԭ��Ϊ7��
��ϰ��ϵ�д�
�����Ŀ
 2CaO+O2��+2xH2O���õ�O2�ڱ�״�������Ϊ672 mL������Ʒ��CaO2�����ʵ���Ϊ_____��
2CaO+O2��+2xH2O���õ�O2�ڱ�״�������Ϊ672 mL������Ʒ��CaO2�����ʵ���Ϊ_____�� ��100%
��100%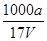 mol��L��1
mol��L��1 mol��L��1
mol��L��1