��Ŀ����
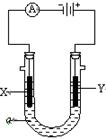
����ͼ��ʾһ�����أ�װ�е��Һa��
X��Y������缫�壬ͨ��������ֱ����Դ��������X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����ش�
 ��1��X���ϵĵ缫��Ӧʽ�� ��
��1��X���ϵĵ缫��Ӧʽ�� ��
��X�������۲쵽�������� ��
��2��Y�缫�ϵĵ缫��ӦʽΪ ������õ缫��Ӧ����ķ����� ��
����Ͽ���ʹ���ﳤ����ɻƽ�ˮ��������Ͽ��բ�ķ���ʴ����ʵ���ֽ��ȵ��������������
��1����բ�����壩��Ҫ�������� ��ʴ������д����ʴ�ĵ缫��Ӧʽ��
������ ��
������ ��
��2���������Ͽ��բ�ķ���ʴ���һ����������飺
��
��1��2H+��2e-��H2����2�֣��� �ų����壬��Һ��졣��1�֣�
��2��2Cl�C��2e-��Cl2����2�֣���
��ʪ��ĵ⻯�ص�����ֽ����ȥ�ܿڣ��۲���ֽ�Ƿ����ɫ����1�֣�
��1��������ʴ��绯ѧ��ʴ��1�֣�
2Fe��4e-��2Fe2+��2�֣� O2+2H2O+4e-��4OH-(2�֣�
��2����ʱˢ���ᣬ�����������������ˮ�Ӵ������ڴ�բ����Ƕп�鲢��ʱ���䣻��ֱ����Դ�ĸ������ӵ���բ�ϣ�������Ϲ��硣
��1�֣����δ�1�����ɣ�

 ��ͼ��ʾһ�����أ����Һ���������ı���NaCl��Һ��X��Y����ʯī�缫��ʵ�鿪ʼǰ�������߸����뼸�η�̪��Һ����
��ͼ��ʾһ�����أ����Һ���������ı���NaCl��Һ��X��Y����ʯī�缫��ʵ�鿪ʼǰ�������߸����뼸�η�̪��Һ����
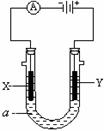
 ��1��ijʵ��С������ͼװ����ȡ������������ش��������⣺
��1��ijʵ��С������ͼװ����ȡ������������ش��������⣺

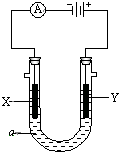
 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺