��Ŀ����
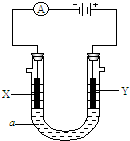
 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һc��A��B������缫�壬ͨ��������ֱ����Դ��������ش��������⣺��1����Ҫ���д�ͭ����Al��Zn��Ag��Pt��Au�����ʣ��ĵ�⾫�������Һcѡ��CuSO4��Һ����
��A�缫�IJ�����
��ͭ
��ͭ
���缫��Ӧʽ��Cu-2e-=Cu2+
Cu-2e-=Cu2+
����˵�������ʷ����ĵ缫��Ӧ����д����������˵����ȷ����
bd
bd
��a������ȫ��ת��Ϊ��ѧ��
b���ڵ�⾫�������У����Һ�а�����Al3+��Zn2+����
c����Һ��Cu2+�������ƶ�
d���������ĵײ��ɻ���Ag��Pt��Au�Ƚ���
��2���ö��Ե缫���CuSO4��Һ������������Cu������Ϊ12.8g���������ϲ����������ڱ�״���µ����Ϊ
2.24
2.24
L����3�����÷�Ӧ2Cu+O2+2H2SO4�T2CuSO4+2H2O���Ʊ�CuSO4�������÷�Ӧ���Ϊ���أ���������Һ����
������Һ
������Һ
��������������ͭ
ͭ
�������缫��ӦʽΪO2+4H++4e-=2H2O
O2+4H++4e-=2H2O
����������1���ٸ��ݵ�������֪��a��������b�Ǹ�������⾫���У���ͭ����������ͭ��������
��a�����ܲ���ȫ��ת��Ϊ��ѧ�ܣ�
b���ڵ�⾫�������У������Ͻ����ŵ磻
c����Һ��Cu2+�������ƶ���
d���������ĵײ��ɻ��ղ����ý�����
��2������ת�Ƶ�����ȼ��㣻
��3���÷�Ӧ�����Է����У�����ֻ����Ƴɵ��أ�ʧ���ӵĽ�������������Ӧ����ʽ����ҺΪ�������Һ�������ϵõ��ӷ�����ԭ��Ӧ��
��a�����ܲ���ȫ��ת��Ϊ��ѧ�ܣ�
b���ڵ�⾫�������У������Ͻ����ŵ磻
c����Һ��Cu2+�������ƶ���
d���������ĵײ��ɻ��ղ����ý�����
��2������ת�Ƶ�����ȼ��㣻
��3���÷�Ӧ�����Է����У�����ֻ����Ƴɵ��أ�ʧ���ӵĽ�������������Ӧ����ʽ����ҺΪ�������Һ�������ϵõ��ӷ�����ԭ��Ӧ��
����⣺��1���ٸ��ݵ�������֪��a��������b�Ǹ�������⾫���У���ͭ����������ͭ��������
A����������A�������Ǵ�ͭ��������ͭʧ���ӷ���������Ӧ���缫��ӦʽΪ��Cu-2e-=Cu2+��
�ʴ�Ϊ����ͭ��Cu-2e-=Cu2+��
��a�����ܲ���ȫ��ת��Ϊ��ѧ�ܣ����в���ת��Ϊ���ܣ��ʴ���
b���ڵ�⾫�������У������Ͻ����ŵ磬���Ե��Һ�а�����Al3+��Zn2+����������ȷ��
c����Һ��Cu2+�������ƶ����ʴ���
d���������ĵײ��ɻ��ղ����ý�������Ag��Pt��Au������ȷ��
��ѡbd��
��2���������ͭ��Һʱ�����������������ӷŵ磬����1mol������Ҫ4mol���ӣ�����ת�Ƶ�����ȵ������ϲ����������ڱ�״���µ����=
��22.4L/mol=2.24L��
�ʴ�Ϊ��2.24��
��3���÷�Ӧ�����Է����У�����ֻ����Ƴɵ��أ�ʧ���ӵĽ�������������Ӧ����ʽ����ҺΪ�������Һ�������ϵõ��ӷ�����ԭ��Ӧ�����������÷�Ӧ���Ϊ���أ���������Һ����ϡ���ᣬ������������ ͭ�������缫��ӦʽΪO2+4H++4e-=2H2O��
�ʴ�Ϊ��������Һ��ͭ��O2+4H++4e-=2H2O��
A����������A�������Ǵ�ͭ��������ͭʧ���ӷ���������Ӧ���缫��ӦʽΪ��Cu-2e-=Cu2+��
�ʴ�Ϊ����ͭ��Cu-2e-=Cu2+��
��a�����ܲ���ȫ��ת��Ϊ��ѧ�ܣ����в���ת��Ϊ���ܣ��ʴ���
b���ڵ�⾫�������У������Ͻ����ŵ磬���Ե��Һ�а�����Al3+��Zn2+����������ȷ��
c����Һ��Cu2+�������ƶ����ʴ���
d���������ĵײ��ɻ��ղ����ý�������Ag��Pt��Au������ȷ��
��ѡbd��
��2���������ͭ��Һʱ�����������������ӷŵ磬����1mol������Ҫ4mol���ӣ�����ת�Ƶ�����ȵ������ϲ����������ڱ�״���µ����=
| ||
| 4 |
�ʴ�Ϊ��2.24��
��3���÷�Ӧ�����Է����У�����ֻ����Ƴɵ��أ�ʧ���ӵĽ�������������Ӧ����ʽ����ҺΪ�������Һ�������ϵõ��ӷ�����ԭ��Ӧ�����������÷�Ӧ���Ϊ���أ���������Һ����ϡ���ᣬ������������ ͭ�������缫��ӦʽΪO2+4H++4e-=2H2O��
�ʴ�Ϊ��������Һ��ͭ��O2+4H++4e-=2H2O��
���������⿼���˵��ԭ������ȷ�ж��������ǽⱾ��ؼ���ע�����ת�Ƶ����غ������������������������Ϊ�ѵ㣮

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ
 ��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���
��1����ʵ֤��������Ƴ�ԭ��صķ�Ӧͨ���Ƿ��ȷ�Ӧ�����л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���
 ��I��Li-SOCl2��ؿ����������������õ�صĵ缫���Ϸֱ�Ϊ﮺�̼�����Һ��LiAlCl4-SOCl2����ص��ܷ�Ӧ�ɱ�ʾΪ��4Li+2SOCl2=4LiCl+S+SO2��
��I��Li-SOCl2��ؿ����������������õ�صĵ缫���Ϸֱ�Ϊ﮺�̼�����Һ��LiAlCl4-SOCl2����ص��ܷ�Ӧ�ɱ�ʾΪ��4Li+2SOCl2=4LiCl+S+SO2�� ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������