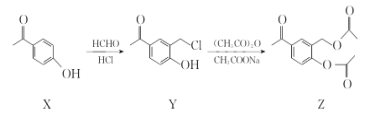
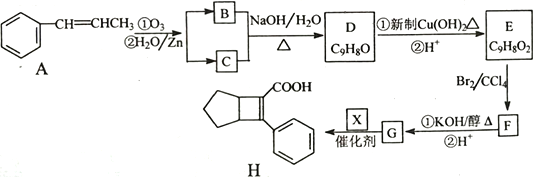
��Ŀ����
����Ŀ��ͭ�Ļ������ڹ�ũҵ�����ͻ�ѧʵ�����ж�������Ҫ�����á���ҵ�ϳ��ð����ʹ��������ͭ{[Cu(NH3)2]Ac}�Ļ����Һ������һ����̼�����������CH3COO��дΪAc������Ӧ����ʽΪ��[Cu(NH3)2]Ac+CO+NH3![]() [Cu(NH3)3CO]Ac��
[Cu(NH3)3CO]Ac��
��1��Cu2+��̬��������Ų�ʽΪ_________��
��2���÷�Ӧ�к��еĵڶ����ڷǽ���Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________��
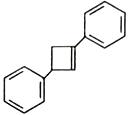
��3��CH3COOH������Cԭ�ӵ��ӻ�����Ϊ__________��
��4�������[Cu(NH3)3CO]Ac���������ӵ���λ��Ϊ________��д��һ����NH3���ӻ�Ϊ�ȵ�����������ӵĻ�ѧʽ____________��
��5��Aԭ�ӵļ۵����Ų�ʽΪ3s23p5��ͭ��A�γɻ�����ľ�����ͼ��ʾ���������ͭԭ�ӣ����þ���Ļ�ѧʽΪ_____________��

���𰸡�1s22s22p63s23p63d9 N��O��C sp3��sp2 4 H3O+ CuCl
��������
��1�����ݹ���ԭ�������ܼ������ӵ͵������У�Ȼ��ͬһ�ܲ���ܼ�������һ��д��Cuԭ�ӵĵ����Ų�ʽ������������ʧȥ���ӣ��õ�Cu2+�ĵ����Ų�ʽ��
��2���÷�Ӧ�к��еĵڶ����ڷǽ���Ԫ��ΪC��N��O������ͬ���ڴ������ң�Ԫ�صĵ�һ�����������ϳ���������ƣ������ж�Ԫ�صĵ�һ�����ܴ�С����ע��ڢ�A�͵ڢ�A��Ԫ��ԭ�ӵ��������ӷֱ�Ϊȫ�����Ͱ����״̬�����һ�����ܴ���ͬ��������Ԫ�أ��ݴ˿��ж�����Ԫ�صĵ�һ�����ܴ�С��ϵ��
��3�������ӻ������=����ԭ�ӵŵ��ӶԵĶ���+����ԭ�ӵ��������������жϣ�
��4���������[Cu(NH3)3CO]Ac�ķ���ʽ��֪��CuΪ����ԭ�ӣ��ṩ�չ����NH3��COΪ���壬�ɷ���ʽ��֪��λ��Ϊ4��ԭ��������ͬ���۵���������ͬ�ķ��ӻ����ӻ�Ϊ�ȵ����壬�ݴ˿�д��NH3�ĵȵ����塣
��5������Aԭ�ӵļ۵����Ų�ʽ3s23p5�ƶϳ�AΪClԭ�ӣ���������̯������������ó�����Ļ�ѧʽ��
��1��ͭԪ��Ϊ29��Ԫ�أ�ԭ�Ӻ�����29�����ӣ�ʧȥ2�������γ�Cu2+������Cu2+�ĵ����Ų�Ϊ1s22s22p63s23p63d9����Ϊ��1s22s22p63s23p63d9��
��2���÷�Ӧ�еڶ����ڵķǽ���Ԫ��Ϊ̼������������Ԫ�أ�����ͬһ���ڴ����ҵ�һ�����ܳ��������ƣ�����Ԫ��ԭ��2p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʴ�Ϊ��N��O��C��
��3������CH3����ԭ���γ�4���������ӻ������ĿΪ4�����õ���sp3�ӻ����Ȼ���COOH����̼ԭ���γ�3���������ӻ������ĿΪ3�����õ���sp2�ӻ���
��4�������[Cu(NH3)3CO]Ac����ΪNH3��CO����λ��Ϊ4��NH3�ĵȵ�������ݵȵ���ԭ������д����ΪH3O+����Ϊ��4��H3O+��
��5��Aԭ�ӵļ۵����Ų�ʽΪ3s23p5��A��Cl��ͭ��Cl�γɻ�����ľ�����ͼ��ʾ������ͭȫ���ھ����У�����4������ԭ�ӵĸ�����![]() ���þ���Ļ�ѧʽΪCuCl����Ϊ��CuCl��
���þ���Ļ�ѧʽΪCuCl����Ϊ��CuCl��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�