��Ŀ����
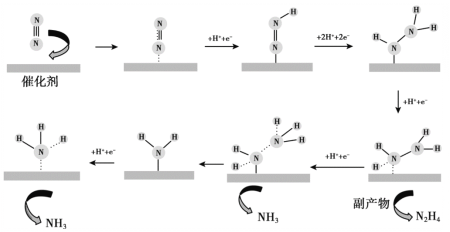
����Ŀ��������Ա�о���ͨ��������N2�ڴ���Au(��)��������NH3�ķ�Ӧ����Ӧ������ͼ��ʾ������˵������ȷ����
A.����ת���������漰�Ǽ��Լ��Ķ��Ѻͼ��Լ�������
B.����NH3���ܵ缫��ӦʽΪ��N2 + 6H+ + 6e- = 2NH3
C.��1 mol N2�ڵ��ص�����������Ӧʱ���ɵõ�2 mol NH3
D.ʹ��Au���������Խ��ͷ�Ӧ�Ļ�ܣ��Ӷ����ѧ��Ӧ����
���𰸡�C
��������
A��ͨ������ʾ��ͼ��֪����Ӧ������N2�����ڵķǼ��Լ����ѣ�������NH3��N2H4�����ڵ�N-H���Լ���A����ȷ��
B��ͨ������ʾ��ͼ��֪����һ��N2����ת��Ϊ����NH3������Ҫ��6�����ӣ����ң���Ҫ6��H+Ҳ���뷴Ӧ�������ܵ缫��ӦʽΪ��![]() ��B����ȷ��
��B����ȷ��
C��ͨ������ʾ��ͼ��֪��N2��ת��ΪNH3�Ĺ����л����ɸ�����N2H4����ˣ�����1molN2�����ܵõ�2molNH3��C�����
D����������ͨ�����ͷ�Ӧ�����߷�Ӧ���ʣ�D����ȷ��
��ѡC��

����Ŀ��ʵ��С���Ʊ��������![]() ��̽�������ʡ�
��̽�������ʡ�
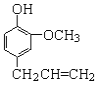
���ϣ�![]() Ϊ��ɫ���壬����
Ϊ��ɫ���壬����![]() ��Һ������ǿ�����ԣ������Ի�������Һ�п��ٲ���
��Һ������ǿ�����ԣ������Ի�������Һ�п��ٲ���![]() ���ڼ�����Һ�н��ȶ���
���ڼ�����Һ�н��ȶ���
(1)�Ʊ�![]() (�г�װ����)
(�г�װ����)
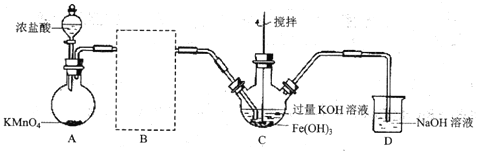
��AΪ��������װ�á�A�з�Ӧ�����ӷ���ʽΪ_________(�̱���ԭΪ![]() )��
)��
������B�����ڽ�����װ�ò��������������������Լ�__________��
��![]() �еõ���ɫ�������Һ��
�еõ���ɫ�������Һ��![]() ����Ҫ��Ӧ�Ļ�ѧ����ʽΪ___________��
����Ҫ��Ӧ�Ļ�ѧ����ʽΪ___________��
(2)̽��![]() ������
������
��ȡC����ɫ��Һ������ϡ���ᣬ��������ɫ���壬����Һa�������������к���![]() ��Ϊ֤���Ƿ�
��Ϊ֤���Ƿ�![]() ������
������![]() ������
������![]() ��������·�����
��������·�����
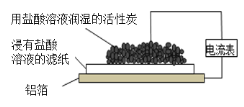
������ | ȡ����a���μ� |
������ | ��KOH��Һ���ϴ��C�����ù��壬����KOH��Һ�� |
�ɷ���������Һ����֪a�к��е�����Ϊ____���������ӵIJ��������ж�һ����![]() ��
��![]() �����������ӻ�������_______����(�����ӷ���ʽ��ʾ)��
�����������ӻ�������_______����(�����ӷ���ʽ��ʾ)��
�ڸ���![]() ���Ʊ�ʵ��ó���������
���Ʊ�ʵ��ó���������___________
![]() (���������)����������ʵ�������
(���������)����������ʵ�������![]() ��
��![]() ��������ǿ����ϵ�෴��ԭ����________________��
��������ǿ����ϵ�෴��ԭ����________________��
�����ϱ�����������Һ�е�������![]() ����֤ʵ�����£�����Һ
����֤ʵ�����£�����Һ![]() ����
����![]() ������
������![]() �Ļ����Һ�У�����Һ��dz��ɫ���������ܷ�֤��������
�Ļ����Һ�У�����Һ��dz��ɫ���������ܷ�֤��������![]() �����ܣ���˵�����ɣ������ܣ���һ�����ʵ�鷽�������ɻ���_______________��
�����ܣ���˵�����ɣ������ܣ���һ�����ʵ�鷽�������ɻ���_______________��
����Ŀ��������������(Na2S2O4)��ӡˢ��ҵ��Ҫ�Ļ�ԭ����������ˮ���������Ҵ����������(K2FeO4)��һ����ɫ����������ˮ��������ij����С������������(��Ҫ�ɷ���FeS2)Ϊ��Ҫԭ���Ʊ������������ƺ�����أ����������������(���ֲ����������ȥ)��
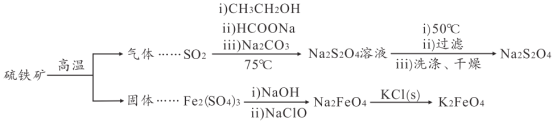
(1)FeS2�к���S22-���ӣ������ʽΪ_____��������������Ҫ�ɷ���O2��SO2��N2���ο����е����ݣ�������ҵ�����������������ᴿ���������ʵ�鷽����_____
�۵�/�� | �е�/�� | �ܶ�/gL-1(��״��) | |
O2 | -218 | -183 | 1.429 |
SO2 | -76 | -10 | 2.9 |
N2 | -210 | -196 | 1.25 |
(2)SO2��Na2S2O4�������������뻹ԭ�������ʵ���֮��Ϊ_____���Ƶõ�Na2S2O4��Һ��ȴ��50�棬���ˣ���_____ϴ�ӣ�����Ƶ�Na2S2O4��
(3)Na2FeO4��KC1�������ֽⷴӦ�Ʊ�K2FeO4��˵����ͬ�¶���K2FeO4���ܽ��_____(���� > ������ < ����=��) Na2FeO4���ܽ�ȡ�
(4)��Һ�е�Na2S2O4�ױ������е�������������NaHSO3����Ӧ�����ӷ���ʽΪ_____
(5)���Ʊ�Na2FeO4ʱ�������������ɺ��ɫ���������ת������ɫ��Һ�������� Na2FeO4��
��д�����ɫ����ת��ΪNa2FeO4�Ļ�ѧ����ʽ��_____��
��Ϊ̽��Na2FeO4��Cl2�����������ǿ����ȡ����������ɫ��Һ���Թ��У��μ�����Ũ���ᣬ������ʹʪ���KI������ֽ����ɫ�����壬����˵��Na2FeO4�������Ա�Cl2��������ǿ��������_____��
����Ŀ��ij��ѧʵ��С�������� KMnO4 ��Һ�Ͳ���(H2C2O4)��Һ��Ӧ���о���������Է�Ӧ���ʵ�Ӱ�죬ʵ��������������£�
��� | ʵ����� | ʵ������ |
��һ֧�Թ����ȼ��� 1mL 0.01 mol/L ����KMnO4 ��Һ���ټ��� 1 �� 3mol/L ����� 9 ������ˮ��������1mL 0.1mol/L ������Һ | ǰ 10min ����Һ��ɫ�����Ա仯������ɫ��dz�� 30 min ����Ϊ��ɫ | |
�� | ����һ֧�Թ����ȼ��� 1mL 0.01mol/L ����KMnO4 ��Һ���ټ��� 10 �� 3mol/L ���ᣬ������ 1mL 0.1mol/L ������Һ | 80s ����Һ��ɫ�����Ա仯������ɫѸ�ٱ�dz��Լ150s ����Ϊ��ɫ |
(1)�������������ᷴӦ�����ӷ���ʽ��������__________��
��MnO![]() +��H2C2O4 + �� = ��Mn2+ +�� +��H2O
+��H2C2O4 + �� = ��Mn2+ +�� +��H2O
(2)��ʵ�� I����ɵó��Ľ�����_____��
(3)����ʵ����� 80s ����Һ��ɫѸ�ٱ�dz��ԭ��С������˲��룺�÷�Ӧ�����ɵ� Mn2+ �Է�Ӧ�д����á����ʵ�� ����֤���롣
�� ��ȫʵ���IJ�����
���Թ����ȼ��� 1mL 0.01mol/L ���� KMnO4 ��Һ��_____�������� 1mL 0.1mol/L ������Һ��
�������������Ӧ�۲쵽��ʵ��������________��
����Ŀ���о���ѧ��Ӧ�������仯�����ʱ仯���о���ѧ��Ӧ����Ҫ�Ƕȡ�
��1����ѧ��Ӧ�������仯����Ҫԭ���ǾɵĻ�ѧ�����ѻ�_____�������µĻ�ѧ���γɻ�_____�������������ų���������������
��2�������ȷ���ұ�������䷴ӦΪ��Fe2O3��2Al![]() 2Fe��Al2O3�����ڷ��ȷ�Ӧ����Ӧ���������______��������������������������������������������ڸ÷�Ӧ�У���������1molAl���������Ͽ�����Fe�����ʵ���Ϊ_____mol��
2Fe��Al2O3�����ڷ��ȷ�Ӧ����Ӧ���������______��������������������������������������������ڸ÷�Ӧ�У���������1molAl���������Ͽ�����Fe�����ʵ���Ϊ_____mol��
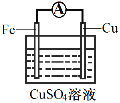
��3��Ϊ̽����Ӧ�����е������仯��ijС��ͬѧ����ͼװ�ý���ʵ�顣
|
|
װ�â� | װ�â� |
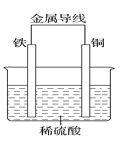
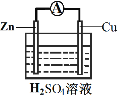
��װ�â��У�Fe��CuSO4��Һ��Ӧ�����ӷ���ʽ��_____��
��װ�â��У������ĵ缫��ӦʽΪ______��
�۹���װ�â�����������ȷ����______������ĸ����
a.H+��Cu���汻��ԭ����������
b.������ZnƬ����������CuƬ
c.���Ӵ�ZnƬ����������CuƬ
d.Zn��Cu�Ķ��ǵ缫���ϣ�Ҳ������缫��Ӧ
��4��ij��ȤС�齫��ȥ����Ĥ��þ��Ͷ�뵽����ϡ�����н���ʵ�飬ʵ���������IJ������ʱ仯�����ͼ������ʾ���Ը����ߵĽ�������ȷ����_____��
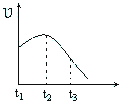
A.��t1��t2��ԭ����þ����ķ�Ӧ�Ƿ��ȷ�Ӧ����ϵ�¶�����
B.��t1��t2��ԭ��ˮ��������ʹ���Ũ������
C.��t2��t3��ԭ�������ŷ�Ӧ�Ľ���þ���������½�
D.��t2��t3��ԭ�������ŷ�Ӧ�Ľ��У�H+��Ũ�����½�