��Ŀ����
����Ŀ���ϳ�����CO+H2���㷺���ںϳ��л����ҵ�ϳ�������Ȼ����ˮ������Ӧ�ȷ�������ȡ�ϳ�����
��1����֪����£�5.6LCH4��ˮ������ȫ��Ӧʱ����51.5KJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ____��
��2����150��ʱ2L���ܱ������У���2 mol CH4��2 mol H2O(g)��ϣ�����15min�ﵽƽ��,��ʱCH4��ת����Ϊ60%���ش��������⣺
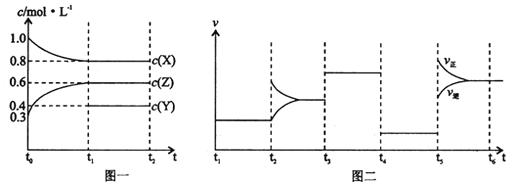
�ٴӷ�Ӧ��ʼ��ƽ�⣬�������ı仯������ʾ�÷�Ӧ����v(H2)=____��
���ڸ��¶��£�����÷�Ӧ��ƽ�ⳣ��K��____��
������ѡ�����ܱ�ʾ�÷�Ӧ�Ѵﵽƽ��״̬����____
A��v(H2)����3v (CO)�� B���ܱ������л��������ܶȲ���
C���ܱ���������ѹǿ���� D��C (CH4) = C (CO)
��3���ϳ����е�����Ҳ���ںϳɰ�����N2 + 3H2![]() 2NH3�������¶Ⱥ�������䣬 �ڼס��ҡ������������н���ƽ��������Ϣ���±���������˵����ȷ����____��
2NH3�������¶Ⱥ�������䣬 �ڼס��ҡ������������н���ƽ��������Ϣ���±���������˵����ȷ����____��
�� �� | ��� | ��ʼ���� | ƽ��ʱNH3�����ʵ��� | ƽ��ʱN2�� ������� | ��Ӧ��ʼʱ������ | ƽ��ʱ������ѹǿ |
�� | 1L | 1molN2+3molH2 | 1.6mol | ���� | ���� | P�� |
�� | 1L | 2molN2+6molH2 | n1 mol | ���� | ���� | P�� |
�� | 2L | 2molN2+6molH2 | n2 mol | span>���� | ���� | P�� |
A��n1=n2=3.2 B������=���������� C������������������ D��P����P��=P��
��4���ϳ����Ƽ��ѵķ�Ӧ����ʽΪ2CO(g) + 4H2(g)![]() CH3OCH3��g��+ H2O(g) ��H = b kJ/mol�����о����ڴ�����Cu��Zn��Al��O��A12O3)�� ѹǿΪ5.OMPa�������£���H2��COֱ���Ʊ����ѣ��������ͼ��ʾ��
CH3OCH3��g��+ H2O(g) ��H = b kJ/mol�����о����ڴ�����Cu��Zn��Al��O��A12O3)�� ѹǿΪ5.OMPa�������£���H2��COֱ���Ʊ����ѣ��������ͼ��ʾ��

�� 290��ǰ��COת���ʺͼ��Ѳ��ʵı仯���Ʋ�һ�µ�ԭ����____��
�� b____0������������������������=����������____��
���𰸡� CH4(g)+H2O(g)=CO(g)+3H2(g) ��H=+206 kJ/mol 0.12mol.L-1.min-1 21.87 AC BD �и���Ӧ���� �� ƽ��������¶ȣ����ʽ���
����������1������£�5.6LCH4�����ʵ���Ϊ![]() =0.25mol����1mol CH4��ˮ������ȫ��Ӧ���ɺϳ����ķ�Ӧ��Ϊ+
=0.25mol����1mol CH4��ˮ������ȫ��Ӧ���ɺϳ����ķ�Ӧ��Ϊ+![]() =++206 kJ/mol�����ݷ�Ӧ����ʽ��֪���÷�Ӧ���Ȼ�ѧ����ʽΪ��CH4(g)+H2O(g)=CO(g)+3H2(g) ��H=+206 kJ/mol��
=++206 kJ/mol�����ݷ�Ӧ����ʽ��֪���÷�Ӧ���Ȼ�ѧ����ʽΪ��CH4(g)+H2O(g)=CO(g)+3H2(g) ��H=+206 kJ/mol��
��2�� CH4(g)+H2O(g)=CO(g)+3H2(g)
��ʼʱ��Ũ�ȣ�mol/L�� 1 1 0 0
�ı��Ũ�ȣ�mol/L�� 0.6 0.6 0.6 1.8
ƽ��ʱ��Ũ�ȣ�mol/L�� 0.4 0.4 0.6 1.8
��v(H2)= ![]() =
=![]() =0.12mol.L-1.min-1����K��
=0.12mol.L-1.min-1����K��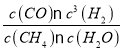 =
=![]() = 21.87mol2L-2����A��v(H2)����3v (CO)�������ݷ�Ӧ����֮�ȵ��ڻ�ѧ������֮����v(H2)����3v (CO)������v(H2)����v(H2)������Ӧ�Ѵﵽƽ��״̬��ѡ��Aѡ��B�����뷴Ӧ�����ʾ�Ϊ���壬��������������䣬��Ӧ�ں��������½��У����ܶ�ʼ�ձ��ֲ��䣬�ܱ������л��������ܶȲ��䣬����˵����Ӧ�Ѵﵽƽ��״̬��ѡ��B��ѡ��C��ͬ��ͬѹ�£������ѹǿ����������ʵ��������ȣ��÷�Ӧ����ӦΪ�����������ķ�Ӧ���ܱ���������ѹǿ���䣬�������ʵ������䣬˵����Ӧ�Ѵﵽƽ��״̬��ѡ��Cѡ�� D����Ӧ��ʼʱ����2 mol CH4��2 mol H2O(g)����Ӧ���������ߵ����ʵ���ʼ�ձ�����ȣ� C (CH4) = C (CO) ����˵����Ӧ�Ѵﵽƽ��״̬��ѡ��D��ѡ����ѡAC����3�����ݱ�������֪���ױ��и���Ӧ���Ũ����ȣ������൱�ڵ�Чƽ�⣬ƽ��ʱN2�����������ȣ����и���Ӧ��Ũ���Ǽ�2������ѹǿ���ڼף�����ѹǿ��ƽ�������������С�ķ����ƶ�������ƽ��ʱ����N2���������С�ڼף�A���ױ��и���Ӧ���Ũ����ȣ�n1= 3.2����ѹǿ���ڼ��ң�ƽ�������ƶ���n2�� 3.2��ѡ��A����B���ױ�Ϊ��Чƽ�⣬ƽ��ʱN2���������������� =��������ѹǿ��ƽ�������ƶ���ƽ��ʱ����N2���������С�ڼף������� =������������ѡ��B��ȷ�� C���ױ��и���Ӧ���Ũ����ȣ�Ϊ��Чƽ�⣬��Ӧ������ȣ�����=���������и���Ӧ��Ũ��ƽ��ʱ�ӽ��ױ��Ķ�������Ӧ���ʽϴ�����������=������ѡ��C����D�������ͬ�������У��ױ���Ч����λ������������ʵ���Ũ����ͬ��ѹǿ���P�� =P��������ƽ��ʱ��λ������������ʵ����ӽ��ױ��Ķ�����P����P�� =P����ѡ��D��ȷ����ѡBD����4���� 290��ǰ��COת�������¶����߶����ͣ����ݷ�Ӧ2CO(g) + 4H2(g)
= 21.87mol2L-2����A��v(H2)����3v (CO)�������ݷ�Ӧ����֮�ȵ��ڻ�ѧ������֮����v(H2)����3v (CO)������v(H2)����v(H2)������Ӧ�Ѵﵽƽ��״̬��ѡ��Aѡ��B�����뷴Ӧ�����ʾ�Ϊ���壬��������������䣬��Ӧ�ں��������½��У����ܶ�ʼ�ձ��ֲ��䣬�ܱ������л��������ܶȲ��䣬����˵����Ӧ�Ѵﵽƽ��״̬��ѡ��B��ѡ��C��ͬ��ͬѹ�£������ѹǿ����������ʵ��������ȣ��÷�Ӧ����ӦΪ�����������ķ�Ӧ���ܱ���������ѹǿ���䣬�������ʵ������䣬˵����Ӧ�Ѵﵽƽ��״̬��ѡ��Cѡ�� D����Ӧ��ʼʱ����2 mol CH4��2 mol H2O(g)����Ӧ���������ߵ����ʵ���ʼ�ձ�����ȣ� C (CH4) = C (CO) ����˵����Ӧ�Ѵﵽƽ��״̬��ѡ��D��ѡ����ѡAC����3�����ݱ�������֪���ױ��и���Ӧ���Ũ����ȣ������൱�ڵ�Чƽ�⣬ƽ��ʱN2�����������ȣ����и���Ӧ��Ũ���Ǽ�2������ѹǿ���ڼף�����ѹǿ��ƽ�������������С�ķ����ƶ�������ƽ��ʱ����N2���������С�ڼף�A���ױ��и���Ӧ���Ũ����ȣ�n1= 3.2����ѹǿ���ڼ��ң�ƽ�������ƶ���n2�� 3.2��ѡ��A����B���ױ�Ϊ��Чƽ�⣬ƽ��ʱN2���������������� =��������ѹǿ��ƽ�������ƶ���ƽ��ʱ����N2���������С�ڼף������� =������������ѡ��B��ȷ�� C���ױ��и���Ӧ���Ũ����ȣ�Ϊ��Чƽ�⣬��Ӧ������ȣ�����=���������и���Ӧ��Ũ��ƽ��ʱ�ӽ��ױ��Ķ�������Ӧ���ʽϴ�����������=������ѡ��C����D�������ͬ�������У��ױ���Ч����λ������������ʵ���Ũ����ͬ��ѹǿ���P�� =P��������ƽ��ʱ��λ������������ʵ����ӽ��ױ��Ķ�����P����P�� =P����ѡ��D��ȷ����ѡBD����4���� 290��ǰ��COת�������¶����߶����ͣ����ݷ�Ӧ2CO(g) + 4H2(g)![]() CH3OCH3��g��+ H2O(g)��֪���������������Ӧ�ý��ͣ�����������֤����������ķ�Ӧ���ɼ��ѣ���CO��ת���ʺͼ��Ѳ��ʵı仯���Ʋ�һ�µ�ԭ�����и���Ӧ�������ڸ���ͼ����Ϣ��֪��ƽ��������¶ȣ����ʽ��ͣ�ƽ�����淴Ӧ�����ƶ����淴ӦΪ���ȷ�Ӧ��������ӦΪ���ȷ�Ӧ����H = b�� 0��
CH3OCH3��g��+ H2O(g)��֪���������������Ӧ�ý��ͣ�����������֤����������ķ�Ӧ���ɼ��ѣ���CO��ת���ʺͼ��Ѳ��ʵı仯���Ʋ�һ�µ�ԭ�����и���Ӧ�������ڸ���ͼ����Ϣ��֪��ƽ��������¶ȣ����ʽ��ͣ�ƽ�����淴Ӧ�����ƶ����淴ӦΪ���ȷ�Ӧ��������ӦΪ���ȷ�Ӧ����H = b�� 0��

 ����5��2���ϵ�д�
����5��2���ϵ�д�