ћвƒњƒЏ»Ё
”–A°ҐB°ҐC°ҐD°ҐE°ҐFЅщ÷÷ґћ÷№∆Џµƒ÷ч„е‘™ЋЎ°Ґ‘≠„”–т э“јіќ‘ціу£ђ∆д÷–A°ҐD‘™ЋЎµƒ‘≠„”„оЌвµз„”≤гЊщ÷ї”–“їЄцµз„”£ђ«“AЇЌDњ…–ќ≥…јл„”їѓЇѕќп£їC°ҐFЌђ÷ч„е£ђB‘™ЋЎќї”Џ‘™ЋЎ÷№∆Џ±н÷–µЏґю÷№∆Џ£ђ«“‘≠„”„оЌв≤гµз„” э’ЉЇЋЌвµз„”„№ эµƒ3/4£ђF‘™ЋЎ‘≠„”„оЌвµз„”≤г±»Ќђ÷№∆ЏµƒE‘™ЋЎ‘≠„”µƒ„оЌвµз„”≤гґа4Єцµз„”£ђ«лїЎір£Ї
£®1£©–і≥цA°ҐDЅљ÷÷‘™ЋЎ„й≥…µƒїѓЇѕќпµƒµз„” љ £ї
£®2£©–і≥цB°ҐD°ҐE»э÷÷‘™ЋЎ„й≥…µƒ≥£ЉыїѓЇѕќпµƒ√ы≥∆ £ї
£®3£©є§“µ…ъ≤ъEµƒЈљЈ® « £ї
£®4£©D°ҐE°ҐF»э‘™ЋЎ„оЄяЉџ—хїѓќпґ‘”¶µƒЋЃїѓќпѕаї•÷ЃЉдЈҐ…ъЈі”¶µƒјл„”Јљ≥ћ љќ™£Ї
°Ґ
°Ґ
°£
£®1£©–і≥цA°ҐDЅљ÷÷‘™ЋЎ„й≥…µƒїѓЇѕќпµƒµз„” љ £ї
£®2£©–і≥цB°ҐD°ҐE»э÷÷‘™ЋЎ„й≥…µƒ≥£ЉыїѓЇѕќпµƒ√ы≥∆ £ї
£®3£©є§“µ…ъ≤ъEµƒЈљЈ® « £ї
£®4£©D°ҐE°ҐF»э‘™ЋЎ„оЄяЉџ—хїѓќпґ‘”¶µƒЋЃїѓќпѕаї•÷ЃЉдЈҐ…ъЈі”¶µƒјл„”Јљ≥ћ љќ™£Ї
°Ґ
°Ґ
°£
£®1£©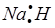 £®2£©∆Ђ¬ЅЋбƒ∆ £®3£©µзљвЈ®
£®2£©∆Ђ¬ЅЋбƒ∆ £®3£©µзљвЈ®
£®4£©H+ + OH- H2O°Ґ
Al(OH)3 + 3H+ Al3+ + 3H2O°Ґ
Al(OH)3 + OH- AlO2- + 2H2O
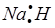 £®2£©∆Ђ¬ЅЋбƒ∆ £®3£©µзљвЈ®
£®2£©∆Ђ¬ЅЋбƒ∆ £®3£©µзљвЈ®£®4£©H+ + OH-
Al(OH)3 + 3H+
Al(OH)3 + OH-
‘ћвЈ÷ќц£ЇA°ҐB°ҐC°ҐD°ҐE°ҐFЅщ÷÷‘™ЋЎЈ÷±рќ™H°ҐO°ҐF°ҐNa°ҐAl°ҐCl°££®1£©A°ҐDЅљ÷÷‘™ЋЎ„й≥…µƒїѓЇѕќпќ™NaH£ї£®2£©B°ҐD°ҐE»э÷÷‘™ЋЎ„й≥…µƒ≥£ЉыїѓЇѕќпќ™NaAlO2£ї£®3£©Alµƒљр ф–‘љѕїо∆√£ђє є§“µ…ѕ”√µзљвЈ®÷∆±Є£ї£®4£©D°ҐE°ҐF»э‘™ЋЎ„оЄяЉџ—хїѓќпґ‘”¶µƒЋЃїѓќпЈ÷±рќ™NaOH°ҐAl£®OH£©3ЇЌHClO4£ї∆д÷–£ђNaOHќ™«њЉо£ђHClO4ќ™«њЋб£ђЋЃ»№“Ї÷–Ќк»Ђµзјл£їAl£®OH£©3ќ™Ѕљ–‘їѓЇѕќп£ђ й–іјл„”Јљ≥ћ љ ±“™–іїѓ—І љ°£
µг∆ј£Ї‘™ЋЎ÷№∆Џєж¬… «ЄяњЉ±ЎњЉ÷™ ґµг£ђњЉ…ъ‘Џ±ЄњЉ÷–”¶јќјќ∞—ќ’Єчєж¬…µƒµЁ±д£ђ≤Ґ„Ґ“вїэјџ’∆ќ’ґћ÷№∆Џ‘™ЋЎµƒљбєє”л–‘÷ °£

ЅЈѕ∞≤бѕµЅ–ір∞Є
ѕаєЎћвƒњ
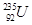 ‘≠„”≥£”√”ЏЇЋЅ—±дЈі”¶£ђѕ¬Ѕ–ґ‘∆д√и ц’э»Јµƒ «
‘≠„”≥£”√”ЏЇЋЅ—±дЈі”¶£ђѕ¬Ѕ–ґ‘∆д√и ц’э»Јµƒ «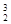 Heњ…“‘„чќ™ЇЋЊџ±д≤ƒЅѕ°£ѕ¬Ѕ–єЎ”Џ
Heњ…“‘„чќ™ЇЋЊџ±д≤ƒЅѕ°£ѕ¬Ѕ–єЎ”Џ