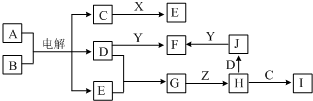
��Ŀ����
Ŀǰ�����϶���õ�������Ȼ��Ƶķ��������������ƣ�2NaCl�����ڣ�
2Na+Cl2������֪����A��B��C��D��E��F������ת����ϵ��
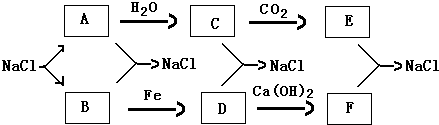
��1���Ը��������仯д��A��B��C��D���ܵĻ�ѧʽ��
A��______B��______C��______D��______E��______F��______��
��2����д��A��A�ڿ�����ȼ�ղ���ֱ���ˮ��Ӧ�Ļ�ѧ����ʽ��
��______��
��______��
��3�������£�B����C��Ӧ��д����Ӧ�����ӷ���ʽ��______��
| ||

��1���Ը��������仯д��A��B��C��D���ܵĻ�ѧʽ��
A��______B��______C��______D��______E��______F��______��
��2����д��A��A�ڿ�����ȼ�ղ���ֱ���ˮ��Ӧ�Ļ�ѧ����ʽ��
��______��
��______��
��3�������£�B����C��Ӧ��д����Ӧ�����ӷ���ʽ��______��
��1�������̿�֪��AΪNa��CΪNaOH��EΪNa2CO3��BΪCl2��DΪFeCl3��FΪCaCl2���ʴ�Ϊ��Na��Cl2��NaOH��FeCl3��Na2CO3��CaCl2��
��2������ˮ�ķ�ӦΪ2Na+2H2O�T2NaOH+H2�����Ƶ�ȼ�ղ���Ϊ�������ƣ���ˮ�ķ�ӦΪ2Na2O2+2H2O�T4NaOH+O2����
�ʴ�Ϊ��2Na+2H2O�T2NaOH+H2����2Na2O2+2H2O�T4NaOH+O2����
��3��������B��C��Ӧ�����ӷ���ʽΪCl2+2OH-�TCl-+ClO-+H2O���ʴ�Ϊ��Cl2+2OH-�TCl-+ClO-+H2O��
��2������ˮ�ķ�ӦΪ2Na+2H2O�T2NaOH+H2�����Ƶ�ȼ�ղ���Ϊ�������ƣ���ˮ�ķ�ӦΪ2Na2O2+2H2O�T4NaOH+O2����
�ʴ�Ϊ��2Na+2H2O�T2NaOH+H2����2Na2O2+2H2O�T4NaOH+O2����
��3��������B��C��Ӧ�����ӷ���ʽΪCl2+2OH-�TCl-+ClO-+H2O���ʴ�Ϊ��Cl2+2OH-�TCl-+ClO-+H2O��

��ϰ��ϵ�д�
�����Ŀ