��Ŀ����
�ٰ뾶ԽС��ԭ���γɵĹ��ۼ�Խ�ι̣�������Խ��____����ܡ����ܡ�����
�ڷǽ�����Խǿ��ԭ���γɵĹ��ۼ�Խ�ι�____����ܡ����ܡ�����
������������ҳ�һЩ���ɣ���д������һ����____����Ԥ��C-Br���ļ��ܷ�Χ ____kJ��mol-1<C-Br����<___kJ��mol-1��
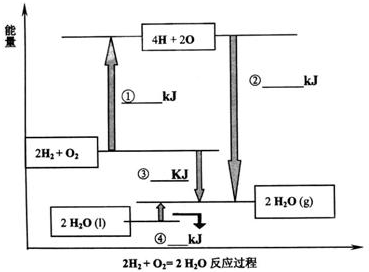
(2)���Ȼ�ѧ����ʽH2 (g)+Cl2 (g)=2HCl(g) ��H= -185kJ��mol-1��������ϱ����ݿ���֪һ����ѧ��Ӧ�ķ�Ӧ�ȣ��跴Ӧ��������Ϊ��̬���뷴Ӧ���������ļ���֮��Ĺ�ϵ��____�� ���Ȼ�ѧ����ʽ
2H2 (g)+S2 (s)=2H2S(g) ��H= -224. skJ��mol-1�ͱ�����ֵ�ɼ����1mol S2 (s)����ʱ��____(����ա��ų���)____kJ��������
218��330
(2)��ѧ��Ӧ�ķ�Ӧ�ȵ���������ļ���֮���뷴Ӧ��ļ���֮�͵IJ�ֵ�� ���գ� 4.5

 �Ƹ������������ϵ�д�
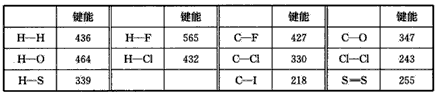
�Ƹ������������ϵ�д���7�֣���ѧ���ļ�����ָ��̬ԭ�Ӽ��γ�1mol��ѧ��ʱ�ͷŵ��������磺H(g)��I(g)��H��I(g)��297KJ ��H��I���ļ���Ϊ297kJ/mol��Ҳ��������Ϊ�ƻ�1mol H��I����Ҫ����297KJ����������ѧ��Ӧ�ķ������Կ��ɾɻ�ѧ�����ƻ����»�ѧ�����γɡ��±���һЩ�������ݡ�����λ��kJ/mol��
| | ���� | | ���� | | ���� |
| H��H | 436 | Cl��Cl | 243 | H��Cl | 432 |
| S��S | 255 | H��S | 339 | C��F | 427 |
| C��Cl | 330 | C��I | 218 | H��F | 565 |
| C��O | 347 | H��O | 464 | Si��Si | 176 |
| Si��O | 460 | O=O | 497 | | |
�Ķ�������Ϣ���ش��������⣺
��1�����ݱ��������ж�CCl4���ȶ��� ������ڡ���С�ڡ���CF4���ȶ��ԡ���Ԥ��C��Br���ļ��ܷ�Χ_________< C��Br���� <__________
��2��������Ϊ��H��O���ļ��ܴ���H��S���ļ��ܣ�����H2O���۷е����H2S���۷е㡣���Ƿ���ͬ���ֹ۵㣿�粻��ͬ����˵����Ľ��͡�
��3����֪H2O(l)��H2O(g) ��H��+44kJ/mol,��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��
��7�֣���ѧ���ļ�����ָ��̬ԭ�Ӽ��γ�1mol��ѧ��ʱ�ͷŵ��������磺H(g)��I(g)��H��I(g)��297KJ ��H��I���ļ���Ϊ297kJ/mol��Ҳ��������Ϊ�ƻ�1mol H��I����Ҫ����297KJ����������ѧ��Ӧ�ķ������Կ��ɾɻ�ѧ�����ƻ����»�ѧ�����γɡ��±���һЩ�������ݡ�����λ��kJ/mol��
|
|
���� |
|
���� |
|
���� |
|
H��H |
436 |
Cl��Cl |
243 |
H��Cl |
432 |
|
S��S |
255 |
H��S |
339 |
C��F |
427 |
|
C��Cl |
330 |
C��I |
218 |
H��F |
565 |
|
C��O |
347 |
H��O |
464 |
Si��Si |
176 |
|
Si��O |
460 |
O=O |
497 |
|
|
�Ķ�������Ϣ���ش��������⣺
��1�����ݱ��������ж�CCl4���ȶ��� ������ڡ���С�ڡ���CF4���ȶ��ԡ���Ԥ��C��Br���ļ��ܷ�Χ_________< C��Br���� <__________
��2��������Ϊ��H��O���ļ��ܴ���H��S���ļ��ܣ�����H2O���۷е����H2S���۷е㡣���Ƿ���ͬ���ֹ۵㣿�粻��ͬ����˵����Ľ��͡�
��3����֪H2O(l)��H2O(g) ��H��+44kJ/mol,��д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��