��Ŀ����
(ÿ��2�֣���10��)A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ������������������Ԫ�غ˵������Ϊ42��B��Cͬ���ڣ�A��Dͬ���塣A��B���γ�����Һ̬��������ң�ԭ�Ӹ����ȷֱ�Ϊ2��1��1��1������������Ϣ�ش��������⣺
�żס����������к��зǼ��Թ��ۼ������ʵĵ���ʽ�� ��CԪ�������ڱ��е�λ���� ��
��C��D�������У��뾶��С���� �������ӷ��ţ���
�ǽ�D�ĵ���Ͷ����У���D��ʧ������������Һ�м���E�ĵ��ʣ���ʱ������Ӧ�Ļ�ѧ����ʽ��______________________________________________________��
��C��D��E��������ӻ�����DxEC6���侧�����������ھ����о��д����Ե���С�ظ���Ԫ���ṹ����ͼ��ʾ��������D�����á��ʾ��λ�������������е���������ڲ���������EC6x�����á��ʾ��λ�ڸ�������Ķ�������ġ��û�����Ļ�ѧʽ�� ��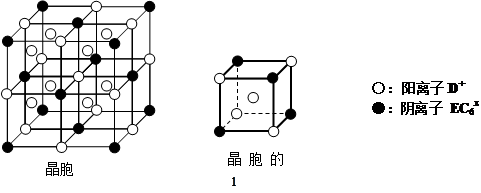
�żס����������к��зǼ��Թ��ۼ������ʵĵ���ʽ�� ��CԪ�������ڱ��е�λ���� ��
��C��D�������У��뾶��С���� �������ӷ��ţ���
�ǽ�D�ĵ���Ͷ����У���D��ʧ������������Һ�м���E�ĵ��ʣ���ʱ������Ӧ�Ļ�ѧ����ʽ��______________________________________________________��
��C��D��E��������ӻ�����DxEC6���侧�����������ھ����о��д����Ե���С�ظ���Ԫ���ṹ����ͼ��ʾ��������D�����á��ʾ��λ�������������е���������ڲ���������EC6x�����á��ʾ��λ�ڸ�������Ķ�������ġ��û�����Ļ�ѧʽ�� ��

�� �ڶ����ڡ��ڢ�A�� �� Na+
�ڶ����ڡ��ڢ�A�� �� Na+
�� 2Al��2NaOH��2H2O��2NaAlO2��3H2�� �� Na3AlF6
 �ڶ����ڡ��ڢ�A�� �� Na+
�ڶ����ڡ��ڢ�A�� �� Na+�� 2Al��2NaOH��2H2O��2NaAlO2��3H2�� �� Na3AlF6
����Ԫ�ص������ڱ��е�λ�ü����ʿ�֪��A��B��C��D��E���Ƕ�����Ԫ�طֱ���H��O��F��Na��Al��
��1��˫��ˮ�к��зǼ��Լ��������ʽΪ ��
��
��2����������Ų���ͬ�����������뾶��ԭ���������������С������������С�ڷ����ӵİ뾶��
��3���ƺ�ˮ��Ӧ�����������ƣ������������Ʒ�Ӧ����������ƫ�����ƺ�ˮ������ʽΪ2Al��2NaOH��2H2O��2NaAlO2��3H2����
��4�����ݾ����Ľṹͼ��֪�����������ӵĸ�����8����1��4��1/8����12�������ӵĸ�����8����4��1/8����4�����������Ӻ������ӵĸ���֮����3�U1������仯ѧʽNa3AlF6��
��1��˫��ˮ�к��зǼ��Լ��������ʽΪ
 ��
����2����������Ų���ͬ�����������뾶��ԭ���������������С������������С�ڷ����ӵİ뾶��
��3���ƺ�ˮ��Ӧ�����������ƣ������������Ʒ�Ӧ����������ƫ�����ƺ�ˮ������ʽΪ2Al��2NaOH��2H2O��2NaAlO2��3H2����
��4�����ݾ����Ľṹͼ��֪�����������ӵĸ�����8����1��4��1/8����12�������ӵĸ�����8����4��1/8����4�����������Ӻ������ӵĸ���֮����3�U1������仯ѧʽNa3AlF6��

��ϰ��ϵ�д�
 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д� ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
 I��
I�� I
I  I
I  I
I