Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°ŅĻ§“Ķ»ľ…’√ļ°Ę Į”ÕĶ»ĽĮ Į»ľŃŌ Õ∑Ň≥ŲīůŃŅĶ™—űĽĮőÔ(NOx)°ĘCO2°ĘCO°ĘSO2Ķ»∆ÝŐŚ£¨—Ō÷ōőŘ»ĺŅ’∆Ý°£∂‘∑Ō∆ÝĹÝ––Õ—Ōű°ĘÕ—ŐľļÕÕ—ŃÚī¶ņŪŅ… ĶŌ÷¬Ő…ęĽ∑Ī£°Ę∑ŌőÔņŻ”√°£
ĘŮ£ģÕ—Ōű£ļ“—÷™£ļH2Ķń»ľ…’»»ő™285.8kJ/mol
ĘŔN2(g)+2O2(g)=2NO2(g) °ųH1=+133kJ/mol
ĘŕH2O(g)=H2O(l) °ųH2=£≠44 kJ/mol
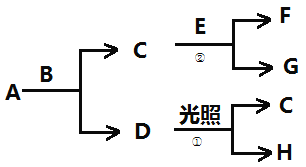
–ī≥Ų‘ŕīŖĽĮľŃīś‘ŕŌ¬£¨H2ĽĻ‘≠NO2…ķ≥…ňģ’Ű∆ÝļÕ∆šňŻőř∂ĺőÔ÷ Ķń»»ĽĮ—ß∑Ĺ≥Ő Ĺő™___°£
ĘÚ£ģÕ—Őľ£ļ
£®1£©ŌÚ2Lļ„»›√‹Ī’»›∆ų÷–ľ”»Ž2molCO2°Ę6molH2£¨‘ŕ ĶĪĶńīŖĽĮľŃ◊ų”√Ō¬£¨∑Ę…ķ∑ī”¶£ļCO2(g)+3H2(g)![]() CH3OH(l) +H2O(l)°£
CH3OH(l) +H2O(l)°£
Ō¬Ń––ū Ųń‹ňĶ√ųīň∑ī”¶īÔĶĹ∆Ĺļ‚◊īŐ¨Ķń «___°£
A£ģĽžļŌ∆ÝŐŚĶń∆Ĺĺý ĹŃŅĪ£≥÷≤ĽĪš B£ģCO2ļÕH2ĶńŐŚĽż∑÷ żĪ£≥÷≤ĽĪš
C£ģCO2ļÕH2Ķń◊™ĽĮ¬ ŌŗĶ» D£ģĽžļŌ∆ÝŐŚĶń√‹∂»Ī£≥÷≤ĽĪš
E£ģ1 molCO2…ķ≥…ĶńÕ¨ Ī”–3molH°™HľŁ∂ŌŃ—
£®2£©‘ŕT1°ś Ī£¨ŐŚĽżő™2LĶńļ„»›»›∆ų÷–≥š»ŽőÔ÷ ĶńŃŅ÷ģļÕő™3molĶńH2ļÕCO£¨∑Ę…ķ∑ī”¶CO(g) +2H2(g)![]() CH3OH(g)∑ī”¶īÔĶĹ∆Ĺļ‚ ĪCH3OHĶńŐŚĽż∑÷ ż£®V%£©”Žn(H2)/n(CO)ĶńĻōŌĶ»ÁÕľňý ĺ°£
CH3OH(g)∑ī”¶īÔĶĹ∆Ĺļ‚ ĪCH3OHĶńŐŚĽż∑÷ ż£®V%£©”Žn(H2)/n(CO)ĶńĻōŌĶ»ÁÕľňý ĺ°£

ĘŔĶĪ∆ū ľn(H2)/n(CO)=2£¨ĺ≠Ļż5minīÔĶĹ∆Ĺļ‚£¨COĶń◊™ĽĮ¬ ő™0.4£¨‘Ú0°ę5minńŕ∆Ĺĺý∑ī”¶ňŔ¬ v(H2)=____°£»Űīň Ī‘ŔŌÚ»›∆ų÷–ľ”»ŽCO£®g£©ļÕCH3OH£®g£©łų0.4mol£¨īÔ–¬∆Ĺļ‚ ĪH2Ķń◊™ĽĮ¬ Ĺę____£®—°ŐÓ°į‘Ųīů°Ī°Ę°įľű–°°ĪĽÚ°į≤ĽĪš°Ī£©£Ľ
ĘŕĶĪ∆ū ľn(H2)/n(CO)=3.5 Ī£¨īÔĶĹ∆Ĺļ‚◊īŐ¨ļů£¨CH3OHĶńŐŚĽż∑÷ żŅ…ń‹ «ÕľŌů÷–Ķń___Ķ„£®—°ŐÓ°įD°Ī°Ę°įE°ĪĽÚ°įF°Ī£©°£
£®3£©“—÷™∑ī”¶A(g)£ęB(g)![]() C(g)£ęD(g)Ķń∆Ĺļ‚≥£ żK÷Ķ”Žő¬∂»ĶńĻōŌĶ»ÁĪŪňý ĺ°£830°ś Ī£¨ŌÚ“ĽłŲ2LĶń√‹Ī’»›∆ų÷–≥š»Ž0.20molAļÕ0.20molB£¨10s ĪīÔĶĹ∆Ĺļ‚°£
C(g)£ęD(g)Ķń∆Ĺļ‚≥£ żK÷Ķ”Žő¬∂»ĶńĻōŌĶ»ÁĪŪňý ĺ°£830°ś Ī£¨ŌÚ“ĽłŲ2LĶń√‹Ī’»›∆ų÷–≥š»Ž0.20molAļÕ0.20molB£¨10s ĪīÔĶĹ∆Ĺļ‚°£
ő¬∂»/°ś | 700 | 830 | 1200 |
K÷Ķ | 1.7 | 1.0 | 0.4 |
ł√∑ī”¶ «____∑ī”¶(ŐÓ°įőŁ»»∑ī”¶°ĪĽÚ°į∑Ň»»∑ī”¶°Ī)£Ľ∑ī”¶≥ű ľ÷Ń∆Ĺ£¨AĶń∆Ĺĺý∑ī”¶ňŔ¬ v(A)£Ĺ_____°£īÔĶĹ∆Ĺļ‚ļů£¨BĶń◊™ĽĮ¬ ő™____°£
°ĺīūįł°Ņ4H2(g)+2NO2(g)£ĹN2(g)+4H2O(g) °ųH=-1100.2kJ/mol DE 0.08mol°§L-1°§min-1 ľű–° F ∑Ň»»∑ī”¶ 0.005mol°§L£≠1°§s£≠1 50%
°ĺĹ‚őŲ°Ņ
ĘŮ“—÷™£ļ«‚∆Ý»ľ…’»»ő™285.8 kJ/mol£¨H2(g)+![]() O2(g)=H2O(l)°ųH=-285.8 kJ/mol
O2(g)=H2O(l)°ųH=-285.8 kJ/mol
ĘŕN2(g)+2O2(g)=2NO2(g)°ųH=+133 kJ/mol
ĘŘH2O(g)=H2O(l)°ųH=-44 kJ/mol
ĘŔ°Ń4-Ęŕ-ĘŘ°Ń4Ķ√ĶĹīŖĽĮľŃīś‘ŕŌ¬£¨H2ĽĻ‘≠NO2…ķ≥…ňģ’Ű∆ÝļÕ∆šňŁőř∂ĺőÔ÷ Ķń»»ĽĮ—ß∑Ĺ≥Ő Ĺő™4H2(g)+2NO2(g)=N2(g)+4H2O(g)°ųH=-1100.2 kJ/mol£Ľ
ĘÚ.(1)A°Ęłýĺ›∑Ĺ≥Ő ĹCO2(g)+3H2(g)£ĹCH3OH(l)+H2O(l)Ņ…÷™ĽžļŌ∆ÝŐŚĶń∆Ĺĺý ĹŃŅ ľ÷’Ī£≥÷≤ĽĪš£¨≤Ľń‹ňĶ√ų∑ī”¶īÔĶĹ∆Ĺļ‚◊īŐ¨£¨Ļ AīŪőů£Ľ
B°ĘŌÚ2L√‹Ī’»›∆ų÷–ľ”»Ž2molCO2°Ę6molH2£¨įī’’1£ļ3∑ī”¶£¨ňý“‘Ļż≥Ő÷–CO2ļÕH2ĶńŐŚĽż∑÷ ż ľ÷’Ī£≥÷≤ĽĪš£¨Ļ BīŪőů£Ľ
C°ĘŌÚ2L√‹Ī’»›∆ų÷–ľ”»Ž2molCO2°Ę6molH2£¨įī’’1£ļ3∑ī”¶£¨CO2ļÕH2Ķń◊™ĽĮ¬ ľ÷’ŌŗĶ»£¨≤Ľń‹»∑∂®∑ī”¶ «∑ŮīÔĶĹ∆Ĺļ‚◊īŐ¨£¨Ļ CīŪőů£Ľ
D°Ę∑ī”¶őÔ «∆ÝŐŚ£¨…ķ≥…őÔ «“ļŐŚ£¨ĽžļŌ∆ÝŐŚĶń√‹∂»Ī£≥÷≤ĽĪš£¨ňĶ√ų∑ī”¶īÔĶĹ∆Ĺļ‚◊īŐ¨£¨Ļ D’ż»∑£Ľ
E°Ę1molCO2…ķ≥…ĶńÕ¨ Ī”–3molH-HľŁ∂ŌŃ—£¨ňĶ√ų’żńś∑ī”¶ňŔ¬ ŌŗÕ¨£¨∑ī”¶īÔĶĹ∆Ĺļ‚◊īŐ¨£¨Ļ E’ż»∑£Ľ
Ļ īūįłő™£ļDE£Ľ
(2)ĘŔH2ļÕCO◊‹Ļ≤ő™3mol£¨«“∆ū ľ![]() =2£¨Ņ…÷™H2ő™2mol°ĘCOő™1mol£¨5minīÔĶĹ∆Ĺļ‚ ĪCOĶń◊™ĽĮ¬ ő™0.4£¨‘Ú£ļ
=2£¨Ņ…÷™H2ő™2mol°ĘCOő™1mol£¨5minīÔĶĹ∆Ĺļ‚ ĪCOĶń◊™ĽĮ¬ ő™0.4£¨‘Ú£ļ
CO(g)+2H2(g)CH3OH(g)
∆ū ľ(mol)£ļ1 2 0
ĪšĽĮ(mol)£ļ0.4 0.8 0.4
∆Ĺļ‚(mol)£ļ0.6 1.2 0.4
»›∆ųĶń»›Ľżő™2L£¨‘Úv(H2)= =0.08mol/(L°§min)£¨ł√ő¬∂»Ō¬∆Ĺļ‚≥£ żK=
=0.08mol/(L°§min)£¨ł√ő¬∂»Ō¬∆Ĺļ‚≥£ żK= £¨īň Ī‘ŔŌÚ»›∆ų÷–ľ”»ŽCO(g)ļÕCH3OH(g)łų0.4mol£¨īň ĪŇ®∂»…ŐQc=
£¨īň Ī‘ŔŌÚ»›∆ų÷–ľ”»ŽCO(g)ļÕCH3OH(g)łų0.4mol£¨īň ĪŇ®∂»…ŐQc=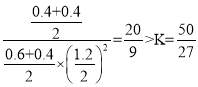 £¨∑ī”¶ŌÚńśŌÚ∑ī”¶ĹÝ––£¨īÔ–¬∆Ĺļ‚ ĪH2Ķń◊™ĽĮ¬ Ĺęľű–°£Ľ
£¨∑ī”¶ŌÚńśŌÚ∑ī”¶ĹÝ––£¨īÔ–¬∆Ĺļ‚ ĪH2Ķń◊™ĽĮ¬ Ĺęľű–°£Ľ
Ę༞ļŌĪ»ņżĶ»”ŕĽĮ—ßľ∆ŃŅ ż÷ģĪ» Ī£¨∆Ĺļ‚ Ī…ķ≥…őÔĶńļ¨ŃŅ◊Óīů£¨Ļ ĶĪ![]() =3.5 Ī£¨īÔĶĹ∆Ĺļ‚◊īŐ¨ļů£¨CH3
=3.5 Ī£¨īÔĶĹ∆Ĺļ‚◊īŐ¨ļů£¨CH3
£®3£©”…ĪŪ÷– żĺ›Ņ…÷™£¨ő¬∂»‘ĹłŖ£¨∆Ĺļ‚≥£ ż‘Ĺ–°£¨ňĶ√ų…żłŖő¬∂»∆Ĺļ‚ŌÚńś∑ī”¶∑ĹŌÚ“∆∂Į£¨…żłŖő¬∂»∆Ĺļ‚ŌÚőŁ»»∑ī”¶∑ĹŌÚ“∆∂Į£¨Ļ ’ż∑ī”¶ő™∑Ň»»∑ī”¶£Ľ…Ť∆Ĺļ‚ Ī≤őľ”∑ī”¶ĶńBĶńőÔ÷ ĶńŃŅő™xmol£¨‘Ú£ļ
A£®g£©+B£®g£©C£®g£©+D£®g£©
Ņ™ ľ£®mol/L£©£ļ0.1 0.1 0 0
ĪšĽĮ£®mol/L£©£ļx x x x
∆Ĺļ‚£®mol/L£©£ļ0.1-x 0.1-x x x
Ļ ![]() =1£¨Ĺ‚Ķ√x=0.05
=1£¨Ĺ‚Ķ√x=0.05
∑ī”¶≥ű ľ÷Ń∆Ĺļ‚£¨AĶń∆Ĺĺý∑ī”¶ňŔ¬ v£®A£©=![]() =0.005molL-1s-1£Ľ∆Ĺļ‚ ĪBĶń◊™ĽĮ¬ ő™
=0.005molL-1s-1£Ľ∆Ĺļ‚ ĪBĶń◊™ĽĮ¬ ő™![]() °Ń100%=50%°£
°Ń100%=50%°£