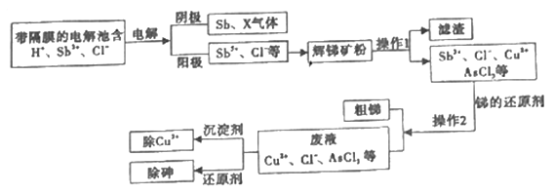
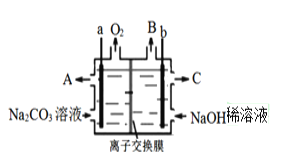
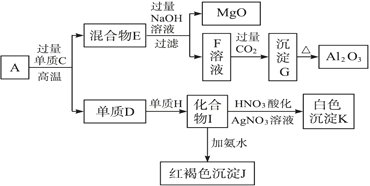
��Ŀ����
����Ŀ��(1)0.5mol/ (NH4)2S��Һ��NH4+ �����ʵ���Ũ��_____________��
(2)��״���£�1.7 g NH3��______L H2S���庬�е���ԭ������ͬ��
(3)��״���£�1.6gij��̬������RO2���Ϊ0.56L������������ʵ���Ϊ____mol��RO2����Է�������Ϊ_______��
(4)MnO2��4HCl(Ũ)![]() MnCl2��Cl2����2H2O�÷�Ӧ�е���������________������4molHCl�μӷ�Ӧ����������HCl�����ʵ�����______mol���練Ӧ��ת����0.4mol���ӣ��������Cl2�ڱ�״�������Ϊ_________L��
MnCl2��Cl2����2H2O�÷�Ӧ�е���������________������4molHCl�μӷ�Ӧ����������HCl�����ʵ�����______mol���練Ӧ��ת����0.4mol���ӣ��������Cl2�ڱ�״�������Ϊ_________L��
(5)��״���£�NH3��CH4��ɵĻ�������ƽ���ܶ�Ϊ0.75g/L���û�������ƽ��Ħ������Ϊ______�������������������ܶ�Ϊ______��
���𰸡�1.0mol/L 3.36L 0.025 64 MnO2 2 4.48 16.8g/mol 8.4
��������
��1������(NH4)2S������ж���Һ��笠����ӵ�Ũ�ȣ�
��2������n��m/M��n��V/Vm��϶��ߵķ�����ɽ��
��3������n��m/M��n��V/Vm�������
��4������Ԫ�ػ��ϼ۽��͵���������������������Ԫ�صĻ��ϼ۱仯�Լ�����ʽ���㱻�������Ȼ�������ɵ����������
��5������M����Vm��ϰ����ӵ������жϡ�
��1��1mol(NH4)2S�����2mol笠����ӣ����0.5mol/L(NH4)2S��Һ��NH4+ �����ʵ���Ũ��Ϊ0.5mol/L��2��1.0mol/L��
��2����״���£�1.7 g NH3�����ʵ�����1.7g��17g/mol��0.1mol������0.3mol��ԭ�ӣ�1�������⺬��2����ԭ�ӣ�����0.3mol H����������ʵ�����0.3mol��2��0.15mol���ڱ���µ����Ϊ0.15mol��22.4L/mol��3.36L��
��3����״���£�1.6gij��̬������RO2���Ϊ0.56L����˸���������ʵ���Ϊ0.56L��22.4L/mol��0.025mol����RO2����Է�������Ϊ1.6��0.025��64��
��4����ӦMnO2��4HCl(Ũ)![]() MnCl2��Cl2����2H2O��MnԪ�ػ��ϼ۽��ͣ�����ԭ����������MnO2������4molHCl�μӷ�Ӧ��ֻ����1mol��������������HCl�����ʵ�����2mol����Ԫ�ػ��ϼ۴ӣ�1�����ߵ�0�ۣ�ʧȥ1�����ӣ��練Ӧ��ת����0.4mol���ӣ��������Cl2��0.4mol��2��0.2mol���ڱ�״�������Ϊ0.2mol��22.4L/mol��4.48L��
MnCl2��Cl2����2H2O��MnԪ�ػ��ϼ۽��ͣ�����ԭ����������MnO2������4molHCl�μӷ�Ӧ��ֻ����1mol��������������HCl�����ʵ�����2mol����Ԫ�ػ��ϼ۴ӣ�1�����ߵ�0�ۣ�ʧȥ1�����ӣ��練Ӧ��ת����0.4mol���ӣ��������Cl2��0.4mol��2��0.2mol���ڱ�״�������Ϊ0.2mol��22.4L/mol��4.48L��
��5����״���£�NH3��CH4��ɵĻ�������ƽ���ܶ�Ϊ0.75g/L���û�������ƽ��Ħ������Ϊ22.4L/mol��0.75g/L��16.8g/mol�����ݰ����ӵ����ɿ�֪��ͬ������������ܶ�֮����Ħ������֮�ȣ�������������������ܶ�Ϊ16.8/2��8.4��

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�