��Ŀ����
����Ŀ��ij�¶��£���Ӧ2A��g��![]() B��g��+C��g����ƽ�ⳣ��Ϊ1�����ݻ�Ϊ2 L���ܱ������м���A��g����20 sʱ��ø���ֵ����ʵ������±���
B��g��+C��g����ƽ�ⳣ��Ϊ1�����ݻ�Ϊ2 L���ܱ������м���A��g����20 sʱ��ø���ֵ����ʵ������±���
�� �� | A��g�� | B��g�� | C��g�� |
���ʵ���/mol | 1.2 | 0.6 | 0.6 |
����˵����ȷ���ǣ� ��
A. ��Ӧǰ20 s��ƽ������Ϊv��A��=0.6 mol��L-1��s-1
B. 20 sʱ������Ӧ���ʵ����淴Ӧ����
C. ��ƽ��ʱ��A��g����ת����Ϊ100%
D. �������¶ȣ�ƽ�ⳣ����Ϊ0.6����Ӧ����H��0
���𰸡�D
��������
A����Ӧǰֻ�з�Ӧ��A�����Է�Ӧǰ20 s������A����0.6��2=1.2 mol��v��A��=![]() =0.03 molL-1s-1����A����
=0.03 molL-1s-1����A����
B��20 sʱ��Q= =0.25����KС����δ�ﵽƽ�⣬��B����
=0.25����KС����δ�ﵽƽ�⣬��B����
C�����淴Ӧ��������ȫת������C����
D�������¶ȣ�ƽ�ⳣ����С��ƽ�����淴Ӧ������У�������Ӧ���ȣ���D��ȷ����ѡD��

����Ŀ��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�졣�ڳ����°������·������ʵ�顣
ʵ���� | ��Ӧ�� | ���� |
�� | 10mL2%H2O2��Һ | �� |
�� | 10mL5%H2O2��Һ | �� |
�� | 10mL5%H2O2��Һ | 1mL0.1mol��L��1FeCl3��Һ |
�� | 10mL5%H2O2��Һ������HCl��Һ | 1mL0.1mol��L��1FeCl3��Һ |
�� | 10mL5%H2O2��Һ������NaOH��Һ | 1mL0.1mol��L��1FeCl3��Һ |
��1��ʵ��ٺ͢ڵ�Ŀ����___��
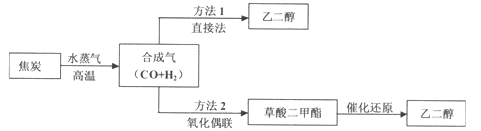
��2��ʵ��ۢܢ��У�������������������ʱ��仯�Ĺ�ϵ��ͼ1������ͼ1�ܹ��ó���ʵ�������__��
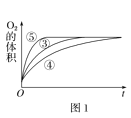
��3������0.1gMnO2��ĩ��50mLH2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ2��ʾ����Ӧ���ʱ仯��ԭ����__��
����Ŀ��ijѧ����0.200 0 mol��L��1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ��������£�
��������ˮϴ�Ӽ�ʽ�ζ��ܣ�������ע��NaOH��Һ����0���̶������ϣ�
�ڹ̶��õζ��ܲ�ʹ�ζ��ܼ��촦����Һ�壻
�۵���Һ������0������0���̶������£������¶�����
����ȡ20.00 mL����Һע��ྻ�Ļ�������������ˮ����ƿ�У�������3�η�̪��Һ�� ���ñ�Һ�ζ����յ㣬���µζ���Һ�������
��ش��������⣺
��1�����ϲ����д������______(����)�����ⶨ���ƫ�ߣ���ԭ�������________(����ĸ)��
A.���Ʊ���Һ�Ĺ���NaOH�л���KOH���� B.�ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�����������ȷ C.ʢװδ֪Һ����ƿ������ˮϴ��������δ֪Һ��ϴ D.����ı�NaOH��Һ���ʵ���Ũ��ƫ��
��2���жϵζ��յ��������_____________________________________��
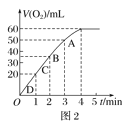
��3����ͼ��ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ________mL��

��4�������������ݣ��������������Ũ�ȣ�________mol��L��1��
�ζ����� | �������(mL) | ���ռ���Һ���(mL) | |
�ζ�ǰ���� | �ζ������ | ||
��һ�� | 20.00 | 0.40 | 20.40 |
�ڶ��� | 20.00 | 2.00 | 24.10 |
������ | 20.00 | 4.00 | 24.00 |
��5����ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�ء�����H2(g)��CO(g)��CH3OH(l)��ȼ������H�ֱ�Ϊ��285.8 kJ��mol��1����283.0 kJ��mol��1�ͣ�726.5 kJ��mol��1����ش��������⣺
����̫���ֽܷ�10 molˮ���ĵ�������________kJ��
��CH3OH(l)����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪ________________��