��Ŀ����
����Ŀ��I.��һ�ܱ������г���1molH2��1molI2��ѹǿΪp(Pa)������һ���¶���ʹ�䷢����Ӧ��H2(g)+I2(g)![]() 2HI(g)��
2HI(g)��
��1�����������ݻ����䣬�����г���1molHe����Ӧ����________�������ӿ�������������������������ͬ����
��2����������������ѹǿ���䣬�����г���1molHe����Ӧ����________��
II.����ɻ�����������ȼ�ϵ����Ϊ������Դ��ȼ�ϵ��һ��ָ����H2��CH4��CO��C2H5OH�ȿ�ȼ������O2һ�ɵĵ��װ�á�����ֱ�ӽ���ѧ��ת��Ϊ���ܣ�����ȼ�ϵ����KOH��ҺΪ����ʣ����ܷ�Ӧ�Ļ�ѧ����ʽΪ2H2+O2��2H2O��
��3�������ϵĵ缫��ӦΪ______________________________��
��4������������ʱ����Һ��c(OH��)_______������������������С����������������
��..A��B��C��D��E��W��T���ֶ���������Ԫ�أ����ǵĺ˵������������A����D��E�γ�10���ӷ��ӡ�Bԭ�ӵ��������������ڴ�����������Cԭ�������������Ǵ�����������2����W��L�������ΪK���M�������֮�ͣ�D��Wͬ���塣�ش��������⣺
��5��Ԫ��B�����ڱ��е�λ����_______________________��
��6��Ԫ��C��ԭ�ӽṹʾ��ͼΪ_______________________��
��7��Ԫ��C��W���γ�CW2��C��T���γ�CT4�������ֻ�����������ܼ��������ʽ�ֱ�Ϊ____________________________________��
��8��Ԫ��A��D��E�γ�10���ӷ��ӵĽṹʽ�ֱ�Ϊ_______________________________��
��9��Ԫ��D��W�ļ��⻯��ķе�ߵ�Ϊ____________���û�ѧʽ��ʾ����
��10��1molCA4��D2��ȫ��Ӧ������������ʱ�ų�����802kJ����÷�Ӧ���Ȼ�ѧ����ʽΪ__________________________________________________________________��
���𰸡����� ���� H2+2OH����2e����2H2O ��С �ڶ�������A�� ![]()
![]() ��
��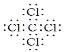 H��O��H��H��F H2O CH4(g)+2O2(g)��CO2(g)+2H2O(g) ��H����802 kJ/mol
H��O��H��H��F H2O CH4(g)+2O2(g)��CO2(g)+2H2O(g) ��H����802 kJ/mol
��������
��..���������Ϣ����Bԭ�ӵ��������������ڴ�������������֪BΪBeԪ�أ�Cԭ�������������Ǵ�����������2������֪CΪCԪ�أ�W��L�������ΪK���M�������֮�ͣ�WΪSԪ�أ�D��Wͬ���壬DΪOԪ�ء�����A����D��E�γ�10���ӷ��ӡ���֪AΪHԪ�أ�EΪFԪ�ء�TԪ�غ˵������W�������ڶ���������Ԫ�أ���TΪClԪ�ء��ݴ˷�����
I.��1������ʱ������ϵ�г���ϡ�����壬��������ѹ������ѹ���䣨������Ũ�Ȳ��䣩����Ӧ���ʲ��䣬��Ϊ�����䣻
��2����ѹʱ������ϵ�г���ϡ�����壬��������ϵ����������ʵ�Ũ�ȼ�С����Ӧ���ʼ�������Ϊ��������
II.��3��ԭ����У���������������Ӧ���������ȼ�ϵ�ص��ܷ�Ӧ����֪�������ϵĵ缫��ӦΪ��H2+2OH����2e����2H2O����Ϊ��H2+2OH����2e����2H2O��
��4�����ݵ�ص��ܷ�Ӧ��֪����ԭ��ع��������в��ϲ���H2O���൱��ϡ�͵���ʣ���Һ��c(OH-)��С����Ϊ����С��
��..��5���ɷ�����֪��Ԫ��BΪBeԪ�أ�λ��Ԫ�����ڱ��еڶ����ڵڢ�A�塣��Ϊ���ڶ�������A�壻
��6��CΪCԪ�أ���ԭ�ӽṹʾ��ͼΪ��![]() ����Ϊ��
������![]() ��
��
��7���ɷ�����֪��CW2��CT4�ֱ�ΪCS2��CCl4��CS2��������CO2�������ʽΪ��![]() ��Clԭ����Cԭ��ͨ�����õ��Ӷ��γ�4�����ۼ��������ʽΪ
��Clԭ����Cԭ��ͨ�����õ��Ӷ��γ�4�����ۼ��������ʽΪ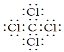 ����Ϊ��
������![]() ��
��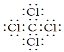 ��
��
��8��Ԫ��A��D��E�γ�10���ӷ��ӷֱ�Ϊ��H2O��HF��OԪ���������6�����ӣ����γ�2�����ۼ��ﵽ8�����ȶ���ϵ��Fԭ���������7�����ӣ��γ�1�����ۼ��ɴﵽ8�����ȶ���ϵ���ɴ˿ɵ�H2O��HF�Ľṹʽ�ֱ�Ϊ��H��O��H��H��F����Ϊ��H��O��H��H��F��
��9��Ԫ��D��W�ļ��⻯��ΪH2O��H2S��H2O�����д����������H2O��H2S�зе�ߵ�ΪH2O����Ϊ��H2O��
��10���ɷ�����֪��CA4��D2�ֱ�ΪCH4��O2�����߷�����Ӧ��CH4+2O2![]() CO2+2H2O�����������Ϣ��1molCH4��O2��ȫ��Ӧ���Ȼ�ѧ����ʽΪ��CH4(g)+2O2(g)��CO2(g)+2H2O(g) ��H����802 kJ/mol����Ϊ��CH4(g)+2O2(g)��CO2(g)+2H2O(g) ��H����802 kJ/mol��
CO2+2H2O�����������Ϣ��1molCH4��O2��ȫ��Ӧ���Ȼ�ѧ����ʽΪ��CH4(g)+2O2(g)��CO2(g)+2H2O(g) ��H����802 kJ/mol����Ϊ��CH4(g)+2O2(g)��CO2(g)+2H2O(g) ��H����802 kJ/mol��

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�