��Ŀ����
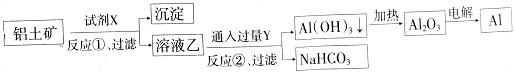
Ϊ�˽��͵��������Ի������ɵ�Ӱ�죬��һ����������·������õ���70��Cu��25��Al��4��Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�
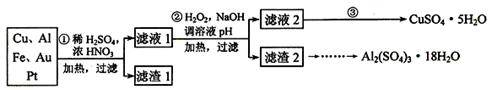
��1���ڢٲ�Cu����ᷴӦ�����ӷ���ʽΪ______________________________���õ�����1����Ҫ�ɷ�Ϊ_________________��
��2���ڢڲ��м���H2O2��������__________________��ʹ��H2O2���ŵ���_________������ҺpH��Ŀ����____________________________________��
��3�������ڢ۲�����Һ2�õ�CuSO4��5H2O�ķ�����_________________________
____________________________________________________________ ��
��4��������2��ȡAl2(SO4)3��18H2O ��������������ַ�����
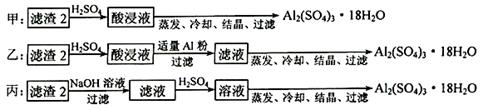
�������ַ����У�_______���������У�ԭ����_______________________________��
��ԭ�������ʽǶȿ��ǣ�_______������������
��5���õζ����ⶨCuSO4��5H2O������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol��L-1 EDTA��H2Y2��������Һ�ζ����յ㣬ƽ������EDTA��Һb mL���ζ���Ӧ���£�Cu2+ + H2Y2���� CuY2�� + 2H+��д������CuSO4��5H2O���������ı���ʽ�أ� __________________ ��

��1���ڢٲ�Cu����ᷴӦ�����ӷ���ʽΪ______________________________���õ�����1����Ҫ�ɷ�Ϊ_________________��
��2���ڢڲ��м���H2O2��������__________________��ʹ��H2O2���ŵ���_________������ҺpH��Ŀ����____________________________________��
��3�������ڢ۲�����Һ2�õ�CuSO4��5H2O�ķ�����_________________________
____________________________________________________________ ��
��4��������2��ȡAl2(SO4)3��18H2O ��������������ַ�����

�������ַ����У�_______���������У�ԭ����_______________________________��
��ԭ�������ʽǶȿ��ǣ�_______������������
��5���õζ����ⶨCuSO4��5H2O������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol��L-1 EDTA��H2Y2��������Һ�ζ����յ㣬ƽ������EDTA��Һb mL���ζ���Ӧ���£�Cu2+ + H2Y2���� CuY2�� + 2H+��д������CuSO4��5H2O���������ı���ʽ�أ� __________________ ��
��1��Cu + 4H+ + 2NO3- =Cu2+ + 2NO2��+ 2H2O��
3Cu + 8H+ + 2 NO3-=3Cu2+ + 2NO��+ 4H2O��2�֣���Au��Pt��1�֣�
��2����Fe2+����ΪFe3+ ��1�֣� ���������ʣ��Ի�������Ⱦ��1�֣���
ʹFe3+��Al3+������ȥ��2�֣�
��3��������Һ2��������������ȴ�� �ᾧ������ �������Ƶ�����ͭ���壨2�֣�
��4���� �����ò�Ʒ�к��н϶�Fe2(SO4)3���ʣ� �� ����1�֣���3�֣�
��5�� �� 100% ��2�֣�
�� 100% ��2�֣�
3Cu + 8H+ + 2 NO3-=3Cu2+ + 2NO��+ 4H2O��2�֣���Au��Pt��1�֣�
��2����Fe2+����ΪFe3+ ��1�֣� ���������ʣ��Ի�������Ⱦ��1�֣���
ʹFe3+��Al3+������ȥ��2�֣�
��3��������Һ2��������������ȴ�� �ᾧ������ �������Ƶ�����ͭ���壨2�֣�
��4���� �����ò�Ʒ�к��н϶�Fe2(SO4)3���ʣ� �� ����1�֣���3�֣�
��5��
 �� 100% ��2�֣�
�� 100% ��2�֣������������1��Cu����ᷴӦ��ʵ������H+��NO3-��Ӧ���淴Ӧ��������Ũ����С���������ӷ���ʽΪCu + 4H+ + 2NO3- =Cu2+ + 2NO2��+ 2H2O��3Cu + 8H+ + 2 NO3-=3Cu2+ + 2NO��+ 4H2O��Au��Pt������ᷴӦ��������������Ҫ�ɷ���Au��Pt��
��2���ӹ��������Ŀ���ǰ��������������������ӣ������ȥ�����Ҽ���������ⲻ�������µ�����������Ⱦ��������Һ��pHĿ����ʹFe3+��Al3+������ȥ
��3������Һ2�õ�CuSO4��5H2O�ķ����ǰ���Һ������Ũ��Һ����ȴ�ᾧ�����˵�����ͭ����
��4�����������У���Ϊ����2����Ҫ�ɷ���Fe��OH��3��Al��OH��3�����������������ȫ���ܽ�ʹ�ƵõIJ�Ʒ�к��н϶�Fe2(SO4)3���ʣ� ��ԭ�������ʽǶȷ������ҷ����������������ܳ�ȥ��������ͬʱ������������������ԭ�������ʽϸ�
��5���ɵζ���Ӧ����ʽ��100ml��Һ��n��Cu2+��=b��10-3��a��5mol,����CuSO4��5H2O��������= b��10-3��a��5��250/a��100%

��ϰ��ϵ�д�
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
�����Ŀ