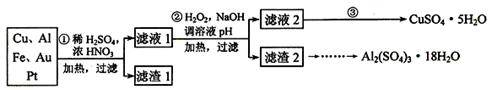
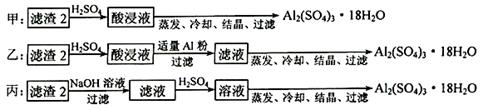
��Ŀ����
������������������������������Ҫ��Ӧ�ã�������ʹ�ø���IJ��Ǵ����������ǺϽ�
��1�������ӷ�Ӧ�ѵ��Ƚ������õ����ƼغϽ��䳣������ ̬��������Һ���̣������Ƶ����ʵ�������Ϊ20%��0.2mol�Ĵ˺Ͻ�ȫ�ؼ��뵽��ˮ��D2O���У���������������������������Ϊ ��
��2��þ���Ͻ��Ǿ������������ʺϽ𡣼�һ��Ͻ��ڿ�����ȼ�գ�������MgO��Al2O3�⣬���п������ɵĵ��������ʵĵ���ʽ�� ����һ��5.1g��þ���Ͻ�Ƭ����3.6 mol��L-1��200ml ��������Һ�У����������1 mol��L-1������������Һ����� mL�������������ٸı䣬��������������0.5mol �ĵ��ӷ���ת�ƣ���Ͻ���Mg�����ʵ�������Ϊ ��
��3������һ��ͭ�ĺϽ�ͭ���ɿ�����Cu��Zn�����ɷֱ������ܷ�����ܷ�����������ֽ��������аѸúϽ�Ͷ�뵽ϡ�����У����ֲ������ݵ��ٶȱ���п�����ᷴӦ���������ٶȿ죬��ԭ���� ��
��Ϊ����ijͭ�Ͻ�ijɷ֣����Ὣ����ȫ�ܽ����NaOH��Һ��pH����pH��3.4ʱ��ʼ���ֳ������ֱ���pHΪ7.0��8.0ʱ���˳����������ͼ��Ϣ�ƶϸúϽ��г�ͭ��һ������ ��
��1�������ӷ�Ӧ�ѵ��Ƚ������õ����ƼغϽ��䳣������ ̬��������Һ���̣������Ƶ����ʵ�������Ϊ20%��0.2mol�Ĵ˺Ͻ�ȫ�ؼ��뵽��ˮ��D2O���У���������������������������Ϊ ��
��2��þ���Ͻ��Ǿ������������ʺϽ𡣼�һ��Ͻ��ڿ�����ȼ�գ�������MgO��Al2O3�⣬���п������ɵĵ��������ʵĵ���ʽ�� ����һ��5.1g��þ���Ͻ�Ƭ����3.6 mol��L-1��200ml ��������Һ�У����������1 mol��L-1������������Һ����� mL�������������ٸı䣬��������������0.5mol �ĵ��ӷ���ת�ƣ���Ͻ���Mg�����ʵ�������Ϊ ��
��3������һ��ͭ�ĺϽ�ͭ���ɿ�����Cu��Zn�����ɷֱ������ܷ�����ܷ�����������ֽ��������аѸúϽ�Ͷ�뵽ϡ�����У����ֲ������ݵ��ٶȱ���п�����ᷴӦ���������ٶȿ죬��ԭ���� ��
��Ϊ����ijͭ�Ͻ�ijɷ֣����Ὣ����ȫ�ܽ����NaOH��Һ��pH����pH��3.4ʱ��ʼ���ֳ������ֱ���pHΪ7.0��8.0ʱ���˳����������ͼ��Ϣ�ƶϸúϽ��г�ͭ��һ������ ��

(1)Һ 0.2NA ��2�� 1440��50%
1440��50%
(3)��ͭп�Ͻ�����γ�ԭ��أ��ӿ컯ѧ��Ӧ���ʡ� ��Al��Ni
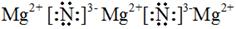 1440��50%
1440��50%(3)��ͭп�Ͻ�����γ�ԭ��أ��ӿ컯ѧ��Ӧ���ʡ� ��Al��Ni
�����������1���ƼغϽ��䳣������Һ̬���Ƶ����ʵ�������Ϊ20%��0.2mol�Ĵ˺Ͻ����ƺͼص����ʵ����ֱ���0.04mol��0.16mol�����ݷ���ʽ2Na��2H2O��2NaOH��H2������֪����ˮ��Ӧ�������������ʵ����ֱ���0.02mol��0.08mol��������0.10mol���������������D2�������к�����������2�������Բ�������������������������Ϊ0.2NA��
��2������þ���뵪����Ӧ���ɵ���þ���������к��д����ĵ��������Ե���������Ӧ���ǵ���þ������þ�����ӻ��������ʽΪ
 �����������ٷ����仯ʱ����ʱǡ�÷�Ӧ����������þ��������������������Һ��ֻ���������ƣ����ʵ�����3.6mol/L��0.2L��0.72mol����ؽ����������غ��֪��������Ҫ�������Ƶ����ʵ�����0.72mol��2��1.44mol������Һ���Ϊ1.44mol��1.0mol/L��1.44L��1440ml����Ͻ���Mg��Al�����ʵ����ֱ�Ϊx��y����2x+3y��0.5mol��24g/mol��x+27g/mol��y��5.1g�����x��y��0.1mol����˺Ͻ���Mg�����ʵ�������Ϊ50%��
�����������ٷ����仯ʱ����ʱǡ�÷�Ӧ����������þ��������������������Һ��ֻ���������ƣ����ʵ�����3.6mol/L��0.2L��0.72mol����ؽ����������غ��֪��������Ҫ�������Ƶ����ʵ�����0.72mol��2��1.44mol������Һ���Ϊ1.44mol��1.0mol/L��1.44L��1440ml����Ͻ���Mg��Al�����ʵ����ֱ�Ϊx��y����2x+3y��0.5mol��24g/mol��x+27g/mol��y��5.1g�����x��y��0.1mol����˺Ͻ���Mg�����ʵ�������Ϊ50%����3��������п�Ľ�����ǿ��ͭ��ͨ�뵽�����У�ͭп�Ͻ�����γ�ԭ��أ��ӿ컯ѧ��Ӧ���ʡ�
����ͼ��֪�����������Ϣ��֪��ʼ���ֳ���ΪAl��OH��3��pH��8.0ʱ���˳�ȥNi��OH��2����˸�ͭ�Ͻ��л���Al��Ni��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 MgO��H2O
MgO��H2O Mg��Cl2��
Mg��Cl2��