��Ŀ����
�״�ȼ�ϵ�����С���ྻ���������������ȸߣ����ڱ�ЯʽͨѶ�豸������������Ӧ�á�ij�״�ȼ�ϵ�ص��ܷ�Ӧʽ2CH4O + 3O2= 2CO2+ 4H2O����ͼ�Ǹ�ȼ�ϵ�ص�ʾ��ͼ������˵����ȷ����
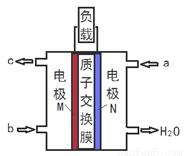
A. a�Ǽ״�ȼ�ϡ�b������
B. ��ת��6mole-ʱ������33.6LO2
C. ������Ӧ��CH4O - 6e- + H2O = CO2��+ 6H+
D. ���Ӵ�N�缫����������Ĥ����M�缫��
 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д���ѧ����ᡢ����������ء��������������ʵ�Ľ�����ȷ��ѡ����
ѡ�� | �������ʵ | ���� |
A | ����������ɱ������ | �����Ļ�ԭ��ʹϸ���ĵ����ʱ��� |
B | ������������ȱ����ƶѪ | �����������������Ȼ��� |
C | ������������������еĹ����� | ����������ǿ��������������������̼ |
D | �ý������Ը�����صĹ�������ˮ�����ʼ� | �������������ˮ���ͷŵĴ������ϩ |
A. A B. B C. C D. D
ij�о���ѧϰС��ͬѧ����NaHCO3��KHCO3��ɵ�ij���Ȼ�������ʵ�飬����������ݣ���������ʵ���Ũ����ȣ�
50mL���� | 50mL���� | 50mL���� | |
m(�����) | 9��2 g | 15��7 g | 27��6 g |
V(CO2)(���) | 2��24 L | 3��36 L | 3��36 L |
�Լ��㣺
��1������������ᷴӦ�ĵ����ӷ���ʽ ��
��2����������ʵ���Ũ�ȣ�
��3��������������ʵ����ʵ���֮�ȡ�

 ������Ҫ��������ά֯���Ⱦɫ��Ҳ������һЩ�л����ϣ�������ʽ���ɫ��B��ͬ���Ұ���(һNH2)������(һNO2)ֱ�����ڱ����ϲ��ʶ�λʱ��ͬ���칹����Ŀ(������ɫ��B)Ϊ
������Ҫ��������ά֯���Ⱦɫ��Ҳ������һЩ�л����ϣ�������ʽ���ɫ��B��ͬ���Ұ���(һNH2)������(һNO2)ֱ�����ڱ����ϲ��ʶ�λʱ��ͬ���칹����Ŀ(������ɫ��B)Ϊ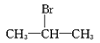 B.
B. 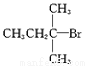 C.
C. 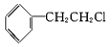 D.
D. 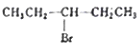
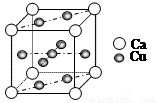
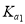 = 1��10-3,
= 1��10-3, 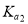 =2��10-8��0. 1 mol . L-1H2TeO3 �ĵ���� a ԼΪ___________ (
=2��10-8��0. 1 mol . L-1H2TeO3 �ĵ���� a ԼΪ___________ ( ); NaHTeO3����Һ��pH_______7(���������=����������
); NaHTeO3����Һ��pH_______7(���������=����������