��Ŀ����
����Ŀ�����������г��ù��������������ⶨ�����̵ĺ�������Ӧԭ��Ϊ��
2Mn2++5S2O82-+8H2O![]() 2MnO4-+10SO42-+16H+
2MnO4-+10SO42-+16H+
(1)�ִ���ѧ�У�������_________�ϵ���������������Ԫ��
(2)�Դӷ��ӵ����幹�ͺ�ԭ�ӵĵ縺�ԡ�����ԭ���ϵŵ��ӶԵȽǶȽ���Ϊʲô��ˮ�ṹʮ�����Ƶ�OF2�ļ��Ժ�С��______________________��
(3)��֪H2S2O8�Ľṹ��ͼ��
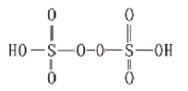
��H2S2O8��ԭ�ӵĹ���ӻ���ʽΪ________________��������Ӧ�б���ԭ��Ԫ��Ϊ_____________��
��S��̬ԭ���е��ӵĿռ��˶�״̬��________________ �֡�
��������Ӧÿ����1 mol MnO4-��S2O82-���ѵĹ��ۼ����ͼ�����ĿΪ___________��__________��
(4)һ�������£�ˮ���Ӽ��ͨ���������H2O���ӽ�ϳ���ά�Ǽܽṹ�����еĶ������Ѩ�пɰ�������С���ӣ��γ�����ˮ�ϰ����ᄃ�塣
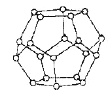
����ͼ��һ����ˮ���ӹ��ɵ���ʮ������Ǽ�(��o����ʾˮ����)��������������Ϊ__________��
��ʵ���ñ��������������Ϊ18.8kJ��mol-1���������ۻ���Ϊ5.0kJ��mol-1����ԭ�������__________��
(5)���ľ����������ǣ��������Ŀ�ܵĶ�������������ijһ������������õ��¾�����ԭ��������ھ���ѧ�ϵ���ƽ�Ƶķ�����+ (1/2��1/2��0) (C���ģ�����+ (0��1/2��1/2) (A����)����+ (1/2��0��1/2) (B����)������ƽ����ָ����֮һ��

��I2��_______���A������B����C�������ľ���.
(6)ʯī�����ɲ�״ʯī�����ӡ���ABAB��ʽ�ѻ����ɣ���ͼ��a)��ʾ����������һ��ʯī������������ͼ��b����ʾ��
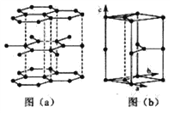
����ͼ�л���������C���ͶӰ���á��� ���̼ԭ��λ�ü��ɣ�_______________��
���̼ԭ��λ�ü��ɣ�_______________��
�ڼ���ʯī�IJ���Ϊ300 pm��C-C����Ϊ150 pm������ʯī������ܶ�Ϊ______g�� cm-3(̼Ԫ�ص��������Ϊ12��NA=6.0��1023������������һλС������
���𰸡� ԭ�ӹ��� OF2��H2O�����幹�����ƣ�ͬΪV�Σ���ˮ���ӵļ��Ժ�ǿ����OF2�ļ���ȴ��С��������Ϊ�� (1)�ӵ縺���Ͽ���������ĵ縺�Բ����������ĵ縺�Բ��2��OF2����ԭ���������Թµ��Ӷԣ������� F-O���й��õ��Ӷ�ƫ��F�������ļ��� sp3 O 9 �Ǽ��Լ���Ҽ� 2.5NA 30 Һ̬ˮ����Ȼ���ڴ������������ڻ�ʱֻ�ƻ��˲�������� B 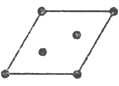 2.3
2.3
��������(1)�ִ���ѧ�У�������ԭ�ӹ����ϵ���������������Ԫ�أ��ʴ�Ϊ��ԭ�ӹ��ף�
(2)�ӵ縺���Ͽ���������ĵ縺�Դ���������ĵ縺�Բ�ֵ��OF2����ԭ���������Թµ��Ӷԣ�������F-O���й��õ��Ӷ�ƫ��F�������ļ��ԣ��Ӷ�����H2O���ӵļ��Ժ�ǿ����OF2���ӵļ���ȴ��С���ʴ�Ϊ���ӵ縺���Ͽ���������ĵ縺�Դ���������ĵ縺�Բ�ֵ��OF2����ԭ���������Թµ��Ӷԣ�������F-O���й��õ��Ӷ�ƫ��F�������ļ��ԣ��Ӷ�����H2O���ӵļ��Ժ�ǿ��
(3)��H2S2O8�У���ԭ�Ӽ۲���Ӷ���=�� �����Ӷ�+����ԭ���ϵŵ��Ӷ�=4+![]() (6-4��1-2)=4�����Բ�ȡsp3�ӻ����÷�Ӧ�У�MnԪ�صĻ��ϼ�����(+2��+7)��OԪ����-2�ۺ�-1�ۣ�����-1��ת��Ϊ-2�ۣ����ϼ۽��ͣ����Ա���ԭ��Ԫ��ΪO���ʴ�Ϊ��sp3�ӻ���O��
(6-4��1-2)=4�����Բ�ȡsp3�ӻ����÷�Ӧ�У�MnԪ�صĻ��ϼ�����(+2��+7)��OԪ����-2�ۺ�-1�ۣ�����-1��ת��Ϊ-2�ۣ����ϼ۽��ͣ����Ա���ԭ��Ԫ��ΪO���ʴ�Ϊ��sp3�ӻ���O��
��S��̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p4������3p4��4������ռ��3����������ӵĿռ��˶�״̬�У�1s��2s��2px��2py��2pz��3s��3px��3py��3pz����9�֣��ʴ�Ϊ��9��
���ɷ�Ӧ��֪��MnԪ�صĻ��ϼ�����(+2��+7)��OԪ�صĻ��ϼ۽���(-1��-2)������10molSO42-ת�Ƶ���10mol���ӣ���ÿ����1 mol MnO4-��ת�Ƶ���5mol���ӣ�S2O8 2-����2.5mol(��2.5NA)O-O��Ǽ��Թ��ۼ����ʴ�Ϊ���Ǽ��Լ���2.5NA��
(4)���ɴ˽ṹ��֪���˵�Ԫ�к���ˮ���ӵĸ���Ϊ��20������ÿ��ˮ�����γɵ��������2����Ԫ������ÿ��ˮ�����γ��������Ϊ�� ![]() �����ܹ��γ������Ϊ��20��
�����ܹ��γ������Ϊ��20��![]() =30���ʴ�Ϊ��30��
=30���ʴ�Ϊ��30��
�ڱ��������������Ϊ18.8kJmol-1�������ۻ���Ϊ5.0kJmol-1��˵�����ۻ�ΪҺ̬ˮʱֻ���ƻ���һ�������������Һ̬ˮ������������ʴ�Ϊ��Һ̬ˮ����Ȼ���ڴ��������
(5)���ľ����������ǣ��������Ŀ�ܵĶ�������������ijһ������������õ��¾�����ԭ�������˵��������İ���ԭ����������ԭ����������ƽ�����IJ�����þ����ǽṹ��Ԫ��2���塣A��B��C��������ķ�����һ�µ�(�ο�ͼ�е�����ϵ)����ֻ�н�������ܵĶ�������ac������ģ������¾�������ԭ�������������bc���Ļ�ab���ģ��õ����¾����е�ԭ�����겻ͬ��ԭ��������˲�����A��Cƽ�ƣ��ʵ���B���ģ���ѡB��
(6)�ٸ���ͼ(b)��֪����C���ͶӰ�����Կ����ı��ε��ĸ������ϸ���1��̼ԭ�ӣ����ͼ(a)����ͼΪ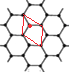 ������һ����һ��̼ԭ��λ�ڸ�ԭ�ӵĶԳ�λ�ã������C���ͶӰΪ
������һ����һ��̼ԭ��λ�ڸ�ԭ�ӵĶԳ�λ�ã������C���ͶӰΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
����ʯī������ÿ��̼ԭ������������������������ÿ����������������̼ԭ�ӣ�����ÿ��̼ԭ��ʵ��ռ�е������ε���ĿΪ0��5����12g̼����1mol̼ԭ�ӣ����Ժ���0.5mol�����Σ���ʯī�����У�ÿ������������12��![]() =2��̼ԭ�ӣ��������������Ϊ
=2��̼ԭ�ӣ��������������Ϊ![]() 1502��300��1030cm3����������
1502��300��1030cm3����������![]() ���Լ�����ܶ�Ϊ
���Լ�����ܶ�Ϊ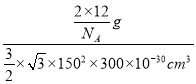 =
=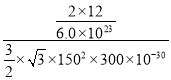 g/cm3=2.3g/cm3���ʴ�Ϊ��/span>2.3��
g/cm3=2.3g/cm3���ʴ�Ϊ��/span>2.3��







