��Ŀ����
����ѧ��--ѡ��3�����ʽṹ�����ʡ���15�֣�
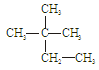
����ͭ������̼��仯�����ڹ�ҵ�����Ź㷺��Ӧ�ã�
��1��Cu2������Χ�����Ų�ͼ�ɱ�ʾΪ ��
��2��Mn��̬ԭ�Ӻ����������ߵ��ܼ��ϵĵ��ӹ��� �ֲ�ͬ���˶�״̬��
��3����ͭ��������Ĵ��£�������CO��������CO2��HCHO��������CO2��H2O
�� N3-��CO2�ǵȵ����壬��N3-�ĽṹʽΪ ��
�� HCHO������Cԭ�ӹ�����ӻ�����Ϊ ��
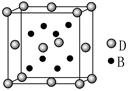
��4����CuSO4��Һ�м������NaOH��Һ������[Cu(OH)4]2���������ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ ��
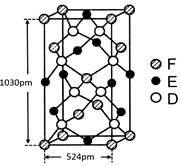
��5�� �þ����x�������䷨���Բ�ð����ӵ��������Խ���ͭ�IJⶨ�õ����½��������Ϊ�����������ܶѻ����߳�Ϊ361pm(��ʾ��3.613=47.05������֪ͭ���ܶ�Ϊ9.00g��cm-3����ͭ������������
g��������λС�����������ӵ�����Ϊ (��ʽ���㣬������λС������
��1����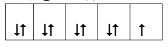 ��2�֣�
��2�֣�
��2��5��2�֣�
3d
��3����[N=N=N]-��2�֣� ��sp2 ��2�֣�
��4��  ��2�֣�
��2�֣�
��5�� 4.23��10-22g��2�֣� M(Cu)=64g/mol= ��NA�� NA=6.05��1023mol-1��3�֣�
��NA�� NA=6.05��1023mol-1��3�֣�
���������������1��Cu2+3d����Ų���9�����ӣ�������Χ�����Ų�ͼ�ɱ�ʾΪ
��2��Mn��̬ԭ�Ӻ���������ߵ��ܼ�Ϊ3d���Ų���5�����ӣ���5�ֲ�ͬ���˶�״̬��
��3����N3-��CO2�ǵȵ����壬��N3-�Ľṹ��CO2���ƣ���ṹ��ʽΪ��[N=N=N]-��
��HCHO������Cԭ���γ���2��̼�ⵥ����1��̼��˫������3���Ҽ�������Cԭ���ӻ���ʽΪsp2
��4��OH?��Oԭ���ṩ�¶Ե��ӣ�Cu�ṩ�չ�����γ���λ��������[Cu(OH)4]2���Ľṹʾ��ͼΪ��
��5��Cu�ľ���Ϊ�����������ܶѻ�������Cuλ�ڶ���������ϣ�1��������Cu��8��1/8+6��1/2=4����ͭ�����������ǣ�4��64�£�6.02��1023��= 4.23��10-22g��һ������Ϊ�о�������M(Cu)=64g/mol= ��NA���ɵ� NA=6.05��1023mol-1��
��NA���ɵ� NA=6.05��1023mol-1��
���㣺���⿼������Ų����ȵ����塢�ӻ���ʽ����λ���������ļ��㡣



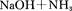
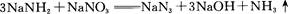 �ش��������⣺
�ش��������⣺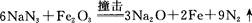

 �۰��� ��18O ��
�۰��� ��18O ��
 ��
��