��Ŀ����
����Ŀ��I.ʵ����������100mL2 mol/LNaOH��Һ����ش���������
��1�����ƹ����в���Ҫʹ�õĻ�ѧ������_______����ѡ�����ĸ����
A.�ձ�. B.100mL����ƿ C.©�� D.��ͷ�ι� E.������
��2����������ƽ��ȡ�����������ƹ���������Ϊ______g��
��.ijУ��ѧ��ȤС��������ͼװ������ȡ����
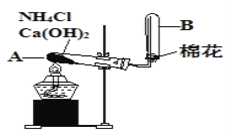
��1��д���ô˷�����NH3�Ļ�ѧ��Ӧ����ʽ___________________��
��2��ͼ���ռ������ķ���Ϊ___________��
��3��������Ũ����IJ����������ռ������ĵ��ܿ����ܹ۲쵽��ʵ������Ϊ___________��
���𰸡� C 8.0 2NH4Cl��Ca(OH)2![]() CaCl2��2H2O��2NH3�� �����ſ����� ���ְ���
CaCl2��2H2O��2NH3�� �����ſ����� ���ְ���
��������������������⿼�����ʵ���Ũ����Һ�����ƣ�������ʵ�����Ʊ���
I.��1���ɹ����������ʵ���Ũ����Һ��ʵ�鲽��Ϊ��������������ܽ����ȴ��ת�ơ�ϴ�ӡ����������ݡ�ҡ�ȡ�װƿ����ǩ����Ҫ�������У���ƽ��ҩ�ס��ձ�����������100mL����ƿ����ͷ�ι�������Ҫ��������©������ѡC��
��2����������ƽ������m��NaOH��=2mol/L![]() 0.1L
0.1L![]() 40g/mol=8.0g��
40g/mol=8.0g��
II.��1��NH4Cl��Ca��OH��2�����Ϲ��ȷ�Ӧ����CaCl2��NH3��H2O����Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca��OH��2![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��2��NH3��������ˮ��NH3���ܶȱȿ���С��ͼ���ռ�NH3�ķ���Ϊ�����ſ�������
��3��Ũ����ӷ�������HCl��NH3���ϳ�NH4Cl��NH4Cl�ǰ�ɫ���壬�����ܹ۲쵽��ʵ������Ϊ���ְ��̡�

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�����Ŀ���������ʵķ����������ȷ����
A | B | C | D | |
ǿ����� | Cu(OH)2 | KCl | HCl | CaCO3 |
������� | NaOH | NH3H2O | BaSO4 | CH3COOH |
�ǵ���� | SO2 | ���ʯ | NH3 | C2H5OH |
�������� | ʯī | ϡH2SO4 | ����KCl | ͭ |
A. A B. B C. C D. D