��Ŀ����
����Ŀ����������Һ����400mL 0.075molL-1��KMnO4��Һ����2gCu2S��CuS�Ļ����������·�Ӧ�� �� 8MnO4- + 5Cu2S + 44H+ = 10Cu2+ + 5SO2��+ 8Mn2+ + 22H2O�� 6MnO4- + 5CuS + 28H+ = 5Cu2+ + 5SO2��+ 6Mn2+ + 14H2O����Ӧ�������Һ���Ͼ�SO2��ʣ���KMnO4ǡ����350mL 0.1molL-1��(NH4)2Fe(SO4)2��Һ��ȫ��Ӧ����֪�÷�Ӧ�Ļ�ѧ����ʽ���£�2KMnO4+10(NH4)2Fe(SO4)2+8H2SO4=K2SO4+2MnSO4+10(NH4)2SO4+5Fe2(SO4)3+8H2O
��1����Ӧ����������Ϊ____����ԭ��Ϊ_______����������Ϊ___________��
��2����˫���ŷ������Ӧ���ĵ���ת�����____ 6MnO4- + 5CuS + 28H+ = 5Cu2+ + 6Mn2++ 5SO2�� + 14H2O
��3����Ӧ����ÿת��3mol���ӣ������ɱ�״������������Ϊ_________L��
��4��KMnO4��Һ��������ﷴӦ��ʣ��KMnO4�����ʵ���Ϊ ______ mol��
���𰸡�MnO4- Cu2S Cu2+��SO2  11.2 0.007
11.2 0.007
��������
��1����Ӧ��8MnO4- + 5Cu2S + 44H+ = 10Cu2+ + 5SO2��+ 8Mn2+ + 22H2O��MnԪ�صĻ��ϼ۽��ͣ���MnO4-Ϊ��������Cu��SԪ�صĻ��ϼ����ߣ�Cu2SΪ��ԭ����ʧȥ���ӱ���������Cu2+��SO2Ϊ�������
����MnO4-��Cu2S��Cu2+��SO2��
��2����Ӧ6MnO4- + 5CuS + 28H+ = 5Cu2+ + 6Mn2++ 5SO2�� + 14H2O�У���Ԫ�ش�+7�۽���Ϊ+2�ۣ��õ�6��5e- ���ӣ���Ԫ����-2�۱�Ϊ+4�ۣ�ʧȥ5��6e- ���ӣ���Ӧ���ĵ���ת�����Ϊ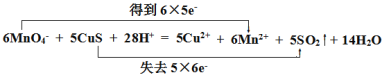 ��
��
����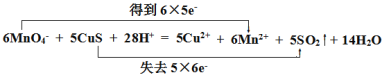 ��
��
��3����Ӧ��6MnO4- + 5CuS + 28H+ = 5Cu2+ + 6Mn2++ 5SO2�� + 14H2O��ÿת��30mol��������5mol SO2����ÿת��3mol���ӣ�����0.5mol SO2����״���µ����Ϊ0.5mol ��22.4L/mol=11.2L��
����11.2��
��4��ʣ���KMnO4ǡ����350mL 0.1molL-1��(NH4)2Fe(SO4)2��Һ��ȫ��Ӧ�����ݷ�Ӧ2KMnO4+10(NH4)2Fe(SO4)2+8H2SO4=K2SO4+2MnSO4+10(NH4)2SO4+5Fe2(SO4)3+8H2O��350mL 0.1molL-1��(NH4)2Fe(SO4)2�����ʵ���=0.1molL-1��350��10-3L=0.035 mol����Ҫ������ص����ʵ���Ϊ![]() ��0.035 mol=0.007 mol��
��0.035 mol=0.007 mol��
����0.007 mol��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�����Ŀ����úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ��ú������
(1)��ˮ����ͨ�����ȵ�̿���ɲ���ˮú������ӦΪC(s)��H2O(g)CO(g)��H2(g)����H����131.3 kJ��mol��1��
�ٸ÷�Ӧ�ڳ�����_______(������������������)�Է����С�
��һ���¶��£���һ���ݻ��ɱ���ܱ������У�����������Ӧ���������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����____(����ĸ����ͬ)��
a�������е�ѹǿ����
b��1 mol H��H�����ѵ�ͬʱ������2 mol H��O��
c��c(CO)��c(H2)
d���ܱ��������ݻ����ٸı�
(2)����ͬ����CO(g)��H2O(g)�ֱ�ͨ�����Ϊ2 L�ĺ����ܱ������У����з�ӦCO(g)��H2O(g)CO2(g)��H2(g)���õ������������ݣ�
ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
H2O | CO | H2 | CO | |||
1 | 650 | 2 | 4 | 1.6 | 2.4 | 6 |
2 | 900 | 1 | 2 | 0.4 | 1.6 | 3 |
3 | 900 | a | b | c | d | t |
��ʵ��1�дӷ�Ӧ��ʼ��ƽ����CO2��ʾ��ƽ����Ӧ����Ϊv(CO2)��________(ȡС�������λ����ͬ)��
�ڸ÷�Ӧ������ӦΪ________(
(3)Ŀǰ��ҵ����һ�ַ�������CO2�������״���һ�������·�����Ӧ��CO2(g)��3H2(g)CH3OH(g)��H2O(g)����ͼ��ʾ�÷�Ӧ���й���������(��λΪkJ��mol��1)�ı仯�������Ϊ1 L�ĺ����ܱ������У�����1 mol CO2��3 mol H2���ﵽƽ������д�ʩ����ʹc(CH3OH)�������________��

a�������¶�
b������He(g)��ʹ��ϵѹǿ����
c����H2O(g)����ϵ�з������
d���ٳ���1 mol CO2��3 mol H2
����Ŀ�����к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��к��������Ӧ�����ʣ��Ը���ʵ��ش�
(1)��ȷ�����õ�2.0 g�ռ���Ʒ���100mL����Һ����Ҫ����Ҫ������������Ͳ���ձ����������⣬�������õ���������_______��________________��
(2)�ü�ʽ�ζ�����ȡ10.00mL����Һ��������ƿ�У�ͬʱ�μ�1-2��ָʾ������ѧ�ϳ�ѡ�õ�ָʾ���з�̪��____________��
(3)��0.2010mol��L-1������ζ������ռ���Һ���ζ�ʱ������ת��ʽ�ζ��ܵIJ������������ֲ�ͣ��ҡ����ƿ������ע��__________________��ֱ���ζ����յ㣬��ѡ���̪��Ϊָʾ�����жϵζ��յ�ı�־��___________________________��
(4)�����������ݣ����c��NaOH��=________���ռ�Ĵ���=____________��
�ζ� ���� | ����Һ��� (mL) | �����������mL�� | |
�ζ�ǰ������mL�� | �ζ��������mL�� | ||
��һ�� | 10.00 | 0.50 | 20.40 |
�ڶ��� | 10.00 | 4.00 | 24.10 |
������ | 10.00 | 1.00 | 24.10 |
��5���Ա���������Һ�ζ�δ֪Ũ�ȵ�����������Һ�����в����������ҺŨ��ƫС���� _______________(�����)��
�ٶ������ζ�ǰƽ�ӣ��ζ����Ӣ�δ�ô���Һ��ϴ��ʽ�ζ���
���ô���Һ��ϴ��ƿ�ܲ�С�Ľ���Һ������ƿ����
�ݵζ��ӽ��յ�ʱ������������ˮ��ϴ��ƿ�ڱ�