��Ŀ����
15��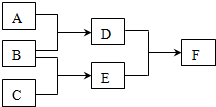 ����A��B��C��D��E��F������ѧ��ѧ�г��������ʣ�����A��B��C���ɶ�����Ԫ����ɵĵ��ʣ�һ��������ת����ϵ���£���Ӧ��������ȥ����
����A��B��C��D��E��F������ѧ��ѧ�г��������ʣ�����A��B��C���ɶ�����Ԫ����ɵĵ��ʣ�һ��������ת����ϵ���£���Ӧ��������ȥ������ش��������⣺
��1�������A��Ԫ�ص�ԭ��M���������K���3�������B��Ԫ�������A��Ԫ������ͬ���壬F����ɫ��ӦΪ��ɫ��
��A��Ԫ�ط�����S��
�����й���D��˵����ȷ����bcd������ĸ����
a��ֻ�л�ԭ�� b����ʹƷ����Һ��ɫ
c����ʹ����ʯ��ˮ����� d����ʹ��ɫʯ����Һ���
��C��E������ˮ��Ӧ�ų����壬C��ˮ��Ӧ�Ļ�ѧ����ʽ��2Na+2H2O�T2NaOH+H2����
��2����B��ԭ�Ӱ뾶��С��ԭ����ɣ�A��������Ư�ۣ�F�����Σ�
����A��Ư�۵Ļ�ѧ����ʽ��2C12+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
����һ��������NO��E��Ӧ������C��ˮ����ת��6mol����ʱ�����������������ʵ���Ϊ3mol��
���� ��1�������A��Ԫ�ص�ԭ��M���������K���3������AӦΪS�����B��Ԫ�������A��Ԫ������ͬ���壬ӦΪO2����DΪSO2��F����ɫ��ӦΪ��ɫ��Ӧ����NaԪ�أ���CΪNa��EΪNa2O2��Na2O��FΪNa2SO4��Na2SO3��
��2����B��ԭ�Ӱ뾶��С��ԭ����ɣ�ӦΪH2��A��������Ư�ۣ�ӦΪCl2����DΪHCl��EΪ�⻯�F�����Σ���FӦΪNH4Cl��EΪNH3��CΪN2���ݴ˽��
��� �⣺��1�������A��Ԫ�ص�ԭ��M���������K���3������AӦΪS�����B��Ԫ�������A��Ԫ������ͬ���壬ӦΪO2����DΪSO2��F����ɫ��ӦΪ��ɫ��Ӧ����NaԪ�أ���CΪNa��EΪNa2O2��Na2O��FΪNa2SO4��Na2SO3��
��A��Ԫ�ط���ΪS���ʴ�Ϊ��S��
��a��DΪSO2�����ϼ۴����м��̬���Ⱦ����������־��л�ԭ�ԣ���a����
b�������������Ư���ԣ���ʹƷ����ɫ����b��ȷ��
c����������������ʯ��ˮ��Ӧ����������Ƴ�������c��ȷ��
d�������������ˮ��Ӧ���������ᣬ�������ԣ���ʹ��ɫʯ����Һ��죬��d��ȷ��
�ʴ�Ϊ��bcd��
������ˮ��Ӧ����������������������Ӧ����ʽΪ��2Na+2H2O�T2NaOH+H2����
�ʴ�Ϊ��2Na+2H2O�T2NaOH+H2����
��2����B��ԭ�Ӱ뾶��С��ԭ����ɣ�ӦΪH2��A��������Ư�ۣ�ӦΪCl2����DΪHCl��EΪ�⻯�F�����Σ���FӦΪNH4Cl��EΪNH3��CΪN2��
��������ʯ���鷴Ӧ�������Ʊ�Ư�ۣ���Ӧ�ķ���ʽΪ��2C12+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O���ʴ�Ϊ��2C12+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O��
�ڷ�Ӧ�ķ���ʽΪ��6NO+4NH3=5N2+6H2O����Ӧ��NOΪ�����������ϼ���+2�۽��͵�0�ۣ���ת��6mol����ʱ�����������������ʵ���Ϊ3mol��
�ʴ�Ϊ��3��
���� ���⿼��������ƶϣ�������ѧ���ķ���������Ԫ�ػ�����֪ʶ���ۺ���������õĿ��飬�ѶȲ���

| A�� | Z=0.5 mol/L | B�� | Z=0.4 mol/L | ||
| C�� | Y2=0.5 mol/L��X2=0.1 mol/L | D�� | X2=0.2 mol/L��Y2=0.6 mol/L |
| A�� | 4 | B�� | 5 | C�� | 6 | D�� | 10 |
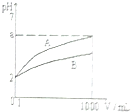 pH=2��A��B��������Һ��1mL���ֱ��ˮϡ�͵�1000mL������Һ��pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
pH=2��A��B��������Һ��1mL���ֱ��ˮϡ�͵�1000mL������Һ��pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | A��B��������Һ���ʵ���Ũ����� | B�� | ϡ�ͺ�A����Һ�����Ա�B����Һ�� | ||
| C�� | ��A=5ʱ��A��ǿ�ᣬB������ | D�� | ��5��a��2����A��B�������� |
| A�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ | |
| B�� | ��ѧ��Ӧ�е������仯ͨ������Ϊ�����ı仯 | |
| C�� | ��ѧ��Ӧ�������仯�Ĵ�С�뷴Ӧ�������������� | |
| D�� | ��ѧ���Ķ��Ѻ��γ��ǻ�ѧ��Ӧ�������仯����Ҫԭ�� |
| A�� | �ۻ�ʱ������ | |
| B�� | �������ӻ�������Ǽ��Թ��ۻ����� | |
| C�� | ˮ��Һ�ĵ��������ܲ� | |
| D�� | ��Һ�����ʷ��Ӻ����ʵ���������ӹ��� |
2KClO3+H2C2O4+H2SO4$\frac{\underline{\;\;��\;\;}}{\;}$2ClO2��+K2SO4+2CO2��+2H2O������˵������ȷ�ǣ�������
| A�� | CO2���������� | |
| B�� | H2C2O4�ڷ�Ӧ�б����� | |
| C�� | H2C2O4��������ǿ��ClO2�������� | |
| D�� | ClO2��ˮ������ʱ����������ǿ������ |
| A�� | 613C �� 612C | B�� | ���ʯ��C60 | C�� | H2��D2 | D�� | CO2�� CO |