��Ŀ����
ijͬѧ��̽��ʳƷ���Ӽ������NH4Al(SO4)2��12H2O���·ֽ�������
��1��Ԥ�������й�����������Ԥ�ⲻ�������� ��
A��NH3��N2��SO2��H2O B��NH3��SO3��H2O
C��NH3��SO2��H2O D��NH3��N2��SO3��SO2��H2O
��2�����Լ��飺ȡһ������������������ʵ��̽�����
�ٰ�ͼʾ��װ���������ȼ������װ�õ������ԣ������� ��
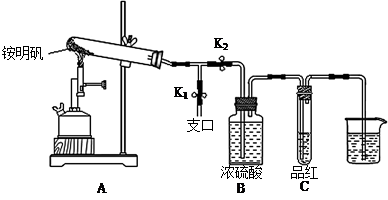
�ڼ�סֹˮ��K1����ֹˮ��K2���þƾ���Ƴ�����ա�ʵ������У�װ��A�͵�����δ������ɫ���壻�Թ�C�е�Ʒ����Һ��ɫ����֧�ڴ��ɼ��鵽NH3�������� ����װ��A��B֮���T�͵����г��ְ�ɫ���壬�ð�ɫ��������� ������һ�����ʵĻ�ѧʽ����
�۷����ó�װ��A�Թ��в����İ�ɫ���������������д��������NaOH��Һ�����ӷ���ʽ ��
��Ϊ�˷�ֹ������ʵ�����ʱ������ ������ĸ��ţ���Ȼ��Ϩ��ƾ���ơ�
A��ȡ���ձ��еĵ��� B����ֹˮ��K1 C���ر�ֹˮ��K2
��3�������ͽ��ۣ�ʵ��֤����������ǣ�1��D�е�5�����塣��ͬ�����²������N2��SO2��������Ƕ�ֵ��V��N2����V��SO2��= ��
��1��Ԥ�������й�����������Ԥ�ⲻ�������� ��
A��NH3��N2��SO2��H2O B��NH3��SO3��H2O
C��NH3��SO2��H2O D��NH3��N2��SO3��SO2��H2O
��2�����Լ��飺ȡһ������������������ʵ��̽�����
�ٰ�ͼʾ��װ���������ȼ������װ�õ������ԣ������� ��

�ڼ�סֹˮ��K1����ֹˮ��K2���þƾ���Ƴ�����ա�ʵ������У�װ��A�͵�����δ������ɫ���壻�Թ�C�е�Ʒ����Һ��ɫ����֧�ڴ��ɼ��鵽NH3�������� ����װ��A��B֮���T�͵����г��ְ�ɫ���壬�ð�ɫ��������� ������һ�����ʵĻ�ѧʽ����
�۷����ó�װ��A�Թ��в����İ�ɫ���������������д��������NaOH��Һ�����ӷ���ʽ ��
��Ϊ�˷�ֹ������ʵ�����ʱ������ ������ĸ��ţ���Ȼ��Ϩ��ƾ���ơ�
A��ȡ���ձ��еĵ��� B����ֹˮ��K1 C���ر�ֹˮ��K2
��3�������ͽ��ۣ�ʵ��֤����������ǣ�1��D�е�5�����塣��ͬ�����²������N2��SO2��������Ƕ�ֵ��V��N2����V��SO2��= ��
��1��C��2�֣�
��2���ٹر�֧�ڿ���K1����K2��1�֣��������ĵ���ͨ��ˮ�У��ȴ��Թܣ�1�֣����������ӵ����г������ݣ�1�֣�����ֹͣ���Ⱥ��ڵ���������һ��ˮ������1�֣���֤�������Ժá�����4�֣�
�ڴ�K1����պ��Ũ����IJ���������֧�ڣ������ְ��̣����߲����Լ���������Ҳ���֣���2�֣�������ʪ����ɫʯ����ֽ���鲻�÷֣���(NH4)2SO4����(NH4)2SO3����SO3(��ʽ�μ��������ʵĻ����Ҳ����)��2�֣�
��Al2O3 +2OH-=2AlO2-+H2O��Al2O3 +3H2O +2OH-=2Al(OH)4-��2�֣���ƽ�����0�֣�
��B��C����BC��2�֣�
��3��1:3��2�֣�
��2���ٹر�֧�ڿ���K1����K2��1�֣��������ĵ���ͨ��ˮ�У��ȴ��Թܣ�1�֣����������ӵ����г������ݣ�1�֣�����ֹͣ���Ⱥ��ڵ���������һ��ˮ������1�֣���֤�������Ժá�����4�֣�
�ڴ�K1����պ��Ũ����IJ���������֧�ڣ������ְ��̣����߲����Լ���������Ҳ���֣���2�֣�������ʪ����ɫʯ����ֽ���鲻�÷֣���(NH4)2SO4����(NH4)2SO3����SO3(��ʽ�μ��������ʵĻ����Ҳ����)��2�֣�
��Al2O3 +2OH-=2AlO2-+H2O��Al2O3 +3H2O +2OH-=2Al(OH)4-��2�֣���ƽ�����0�֣�
��B��C����BC��2�֣�
��3��1:3��2�֣�
�����������1��A������N2��NԪ�ػ��ϼ����ߣ�����SO2��SԪ�ػ��ϼ۽��ͣ�����������ԭ��Ӧԭ����Ԥ�������B������NH3��SO3��H2O��Ϊ��������ԭ��Ӧ��Ԥ�������C������SO2��SԪ�ػ��ϼ۽��ͣ���Ԫ�ػ��ϼ����ߣ�������������ԭ��Ӧԭ����Ԥ�ⲻ������D������Ԫ�ػ��ϼ����ߣ�Ҳ��Ԫ�ػ��ϼ۽��ͣ�Ԥ�������
��2�������ü����������͵�ԭ������װ�õ������ԣ�����Ҫ�ر�֧�ڿ���K1����K2��Ȼ�����ĵ���ͨ��ˮ�У��ȴ��Թܣ��������ӵ����г������ݣ���ֹͣ���Ⱥ��ڵ���������һ��ˮ������֤�������Ժá�
����Ũ�������NH3����K1����պ��Ũ����IJ���������֧�ڣ������ְ��̣�֤������NH3������������ʪ��ʯ����ֽ����Ϊ��������к���SO2��SO3����ֽ�����ܱ�����װ��A��B֮���T�͵����г��ְ�ɫ���������SO2��NH3��Ӧ���ɵ�(NH4)2SO3����SO3��NH3��Ӧ���ɵ�(NH4)2SO4����SO3���壬����ʽ�μ��������ʵĻ���
��A�Թ��в����İ�ɫ�����������������AΪAl2O3����NaOH��Һ��Ӧ�����ӷ���ʽΪ��Al2O3 +2OH-=2AlO2-+H2O��Al2O3 +3H2O +2OH-=2Al(OH)4-��
�ܴ�ֹˮ��K1 ������Һ����֧�ڳ��������ر�ֹˮ��K2�����ܱ��رգ����Է�ֹ�����ķ���Ϊ��B��C����BC��
��3������������ԭ��Ӧ�л��ϼ����ߵ��ܼ����뽵�͵��ܼ�����ȣ��ɵã�6n(N2)=2n(SO2)���ɵ�n(N2)��n(SO2)=1:3����ͬ�������������ʵ���֮�ȵ������֮�ȣ�����V��N2����V��SO2��=1:3��

��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ
 2RAn(�л���)+nH2SO4(ˮ��)��
2RAn(�л���)+nH2SO4(ˮ��)��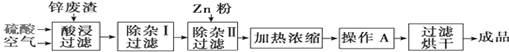
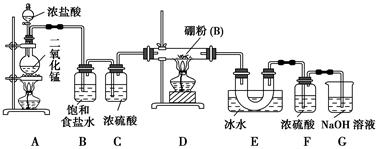
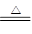 BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��
BCl3����3H2���������������������ƣ�Ҳ��������������Һ��Ӧ��