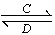��Ŀ����
ijpH��1�Ĺ�ҵ��Һ��ֻ���ܺ������������е������֣�H����Mg2����Ba2����Cl����CO32-��SO42-����ȡ����100 mL��Һ��������ʵ�飺
��һ�ݼ�������AgNO3��Һ���ø������3.50 g��
�ڶ��ݼ�����BaCl2��Һ�ø������2.33 g������������ϴ�ӡ���������������䡣
��������ʵ�飬�����Ʋ���ȷ���ǣ� ��
��һ������Mg2���������ܴ���CO32-����һ������Cl���������ܴ���Ba2���������ܴ���Mg2��
A���٢� B���ڢ� C���ۢ� D���ܢ�
A
�������������������£�CO32-���ܴ��ڣ�����ʵ�飨2����ԭ��ҺӦ��SO42-����Ba2����n(SO42-)��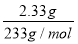 ��0.01 mol��
��0.01 mol��
����ʵ�飨1����һ����Cl����n(Cl��)��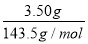 ��0.024 mol
��0.024 mol
���������Ϊ(2��0.01 mol��0.024 mol) NA��mol��1��0.044NA
��H���������������Ϊ0.01NA������Ba2�������ڣ�����һ������Mg2����

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�һ���¶�ʱ����2.0 L�����ܱ������г���2 mol SO2��1 mol O2��������Ӧ��
2SO2(g)��O2(g)  2SO3(g)������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
2SO3(g)������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
t/s | 0 | t1 | t2 | t3 | t4 |
n(SO3)/mol | 0 | 0.8 | 1.4 | 1.8 | 1.8 |
����˵����ȷ���ǣ� ��
A����Ӧ��ǰt1s��ƽ������v(O2)��0.4/t1 mol��L��1��s��1
B�����������������䣬���ѹ����1.0 L��ƽ�ⳣ��������
C����ͬ�¶��£���ʼʱ�������г���4 mol SO3���ﵽƽ��ʱ��SO3��ת���ʴ���10%
D�������¶Ȳ��䣬����������ٳ���2 mol SO2��1 mol O2����Ӧ�ﵽ��ƽ��ʱn(SO3)/n(O2)����